
Page 1 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
alamarBlue
Technical Datasheet
Bio-Rad Laboratories
Endeavour House, Langford Lane,
Kidlington, Oxford, OXON OX5 1GE, UK
Tel: +44 (0) 1865 852700
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc, in certain jurisdictions.
alamarBlue is a trademark of Trek Diagnostic Systems, Inc.
bio-rad-antibodies.com

Page 2 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
alamarBlue
Volume / Quantity: BUF012A – 25 ml
BUF012B – 100 ml
Product Form: Liquid
Preservatives / Stabilizers: None present
Product Description:
alamarBlue is an indicator dye which incorporates an oxidation-reduction (REDOX) indicator that both fluoresces
and changes colour in response to the chemical reduction of growth medium, resulting from cell growth. The
alamarBlue assay is designed to quantitatively measure the proliferation of various human and animal cell lines,
bacteria and fungi.
Indications for Use:
The bioassay can be used to establish proliferation or relative cytotoxicity.
Baseline data for predicting the toxicity of related novel agents can be compared to baseline data with
known in-vivo toxicity.
alamarBlue is for use between pH6.8 and pH7.4.
Product Principle:
Growing cells cause a chemical reduction of alamarBlue.
Continued growth maintains a reduced environment. (fluorescent, red)
Inhibition of growth maintains an oxidized environment. (non-fluorescent, blue)
Data may be collected using either fluorescence-based or absorbance-based instrumentation.
Fluorescence is monitored at 530-560nm excitation wavelength and 590nm emission wavelength.
Absorbance is monitored at 570nm and 600nm.
Shelf life: See datasheet.
Storage and Stability: See datasheet.
This product is light sensitive and should be stored in the dark
Health and Safety information:
The REDOX indicator in alamarBlue has no current or past indication of carcinogenic capacity.
(A full Health and Safety assessment is available upon request)
Manufactured for Bio-Rad by Trek Diagnostic System. U.S. patent 5,501,959.
Please visit bio-rad-antibodies.com/alamarBlue for more:
Calculate results online
Example calculations
References
Frequently asked questions

Page 3 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
QC test for alamarBlue
Materials and Equipment:
0.1M potassium phosphate buffer, pH7.4
10ml test tube
Pipette capable of accurately dispensing 0.4ml
Plate reader with the filters 570nm and 600nm. (Alternative filters can be used – see bottom of page).
Dynatech flat bottom plate
Procedure:
1.
Shake alamarBlue to mix before use
2.
Pipette 0.4ml of alamarBlue into a test tube
3.
Dilute to 10ml with phosphate buffer
4.
Mix well
5.
Pipette 100μl into each well of a clear, flat bottom microplate
6.
Read absorbance at appropriate wavelengths
Wavelength (nm)
Average Absorbance
(Standard Deviation)
540
0.145 (0.002)
570
0.225 (0.003)
600
0.313 (0.004)
630
0.116 (0.002)
Expected results for Quality Control (QC) test – using Dynatech flat bottomed plates.
N.B. Absorbance values may be affected by the type of plate (whether round or flat bottom) and the plate
manufacturer (See Appendix 4 for further information about the effect of plates on absorbance).
No QC protocol is recommended for fluorescence since fluorescence units are arbitrary and the scale used
varies widely from one instrument to another.
For an indication of the absorbance and fluorescence values for the fully oxidized and reduced forms of
alamarBlue with different media, please visit the Frequently Asked Questions section found at:
bio-rad-antibodies.com/alamarBlue.
Alternative wavelengths
Although it is preferable to use the recommended wavelengths, a correction factor can be calculated allowing
the use of one alternative filter (page 8).
Recommended
Alternative
570
540
600
630
570nm and 600nm are the recommended wavelengths; only one wavelength may be altered for its respective
alternative wavelength.

Page 4 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
Method and calculations for alamarBlue
Page
Method for determining optimum length of incubation and plating density.
4
Method for measuring cytotoxicity or proliferation using alamarBlue.
5
Measuring cytotoxicity or proliferation using alamarBlue by Spectrophotometry.
Equation to measure cytotoxicity.
Equation to measure percentage reduction of alamarBlue.
6
6
6
Measuring cytotoxicity or proliferation using alamarBlue by Fluorescence.
Equation to measure cytotoxicity.
Equation to measure percentage reduction of alamarBlue.
7
7
7
Method to measure LD50 using alamarBlue
7
Measuring proliferation using spectrophotometry with different filters.
Equation to measure cytotoxicity.
Equation to measure percentage reduction of alamarBlue.
8
8
8
Method for determining optimum length of incubation and plating density.
The two variables which most affect the response of cells to alamarBlue are length of incubation time and
number of cells plated. It is recommended that the plating density and incubation time be determined for each
cell line using the following procedure:
1.
Harvest cells which are in log phase growth stage and determine cell count. Plate cells at various densities,
above and below the cell density expected to be used.
2.
Aseptically add alamarBlue in an amount equal to 10% of the volume in the well.
3.
Return plates to incubator. Remove the plate and measure fluorescence/absorbance each hour following
plating for the first 6-8 hours. It is also recommended that the plate remain in incubation overnight and
measurements be made the following day at 24 hours. Two kinds of information can be obtained from this
data:
(i)
for any given incubation time selected, the range in cell density relating cell number to
alamarBlue reduction can be determined by a linear response;
(ii)
for any given cell density selected, the incubation time can be determined as the time taken for
the control cells to turn the indicator from the oxidized (blue) form to the fully reduced (red) form.
4.
Measure absorbance at a wavelength of 570nm and 600nm; or measure fluorescence with an excitation
wavelength at 530-560nm and emission wavelength at 590nm.
5.
Calculate the percentage reduction of alamarBlue at each cell density or incubation period.
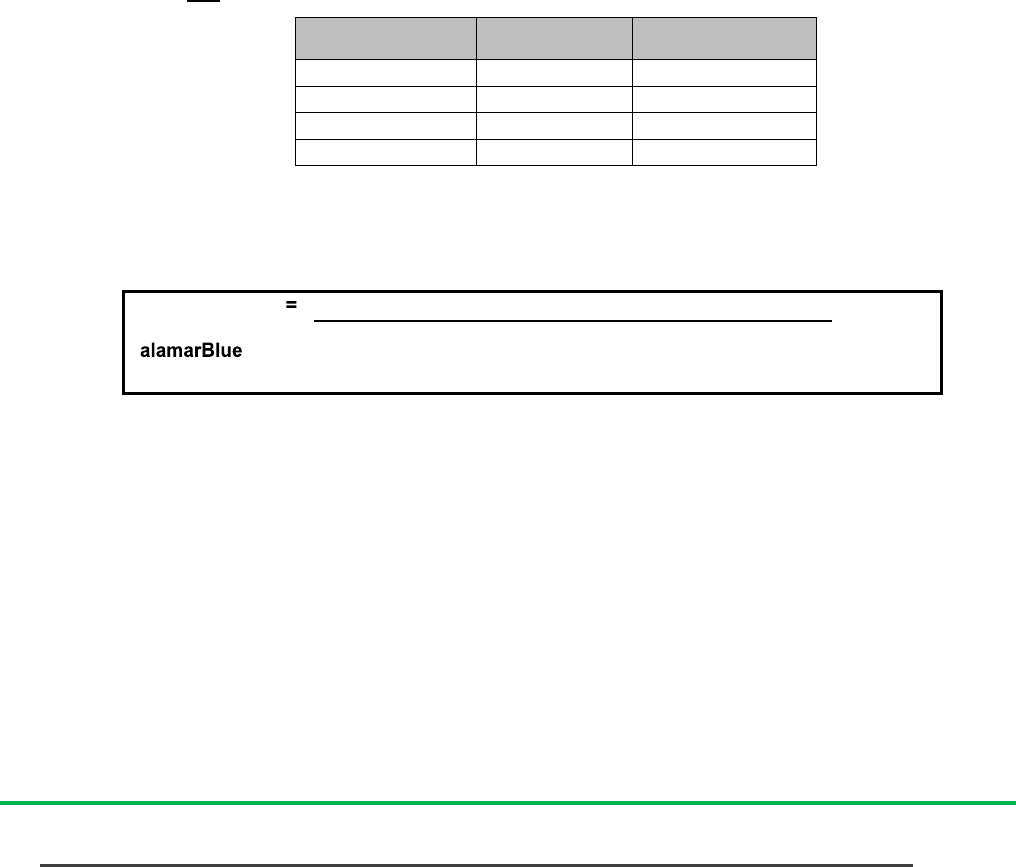
a. Equation 1 calculates percentage reduction of alamarBlue using absorbance
Where: O1 = molar extinction coefficient (E) of oxidized alamarBlue (Blue) at 570nm*
O2= E of oxidized alamarBlue at 600nm*
R1 = E of reduced alamarBlue (Red) at 570nm
R2= E of reduced alamarBlue at 600nm
A1 = absorbance of test wells at 570nm
A2 = absorbance of test wells at 600nm
N1 = absorbance of negative control well (media plus alamarBlue but no cells) at 570nm
x 100
(O2 x A1) - (O1 x A2)
(R1 x N2) - (R2 x N1)
=
Percentage reduction
of alamarBlue
(equation 1)
Page 5 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
N2 = absorbance of negative control well (media plus alamarBlue but no cells) at 600nm
* Only one appropriate substitute wavelength may be used.
Wavelength
Reduced (R)
Oxidized (O)
540nm
104395
47619
570nm
155677
80586
600nm
14652
117216
630nm
5494
34798
Molar extinction coefficients for alamarBlue at different wavelengths
b) To calculate percentage reduction of alamarBlue using fluorescence, use equation 2
Where: FI 590 = Fluorescent Intensity at 590nm emission (560nm excitation).
6.
Plot a graph of percentage reduction of alamarBlue at each cell density or incubation period.
a)
For experiments to determine optimum cell density, plot log of cell density on the x axis, and
percentage reduction on the y axis;
b)
For experiments to determine the optimum incubation time, plot the number of hours on the x axis,
and the percentage reduction on the y axis.
c)
Use the graphs to determine optimum cell density or incubation period.
(Example data is given in appendix 1)
General method for measuring cytotoxicity or proliferation using alamarBlue
1.
Harvest cells which are in the log phase of growth and determine cell count. Adjust the cell count to 1x10
4
cells/ml (suggested cell density). The optimum cell density may vary between cell types.
2.
Plate cells and expose to test agent as determined by researcher.
For determining the effect of a test agent on cell growth, ensure correct controls are included e.g. stimulated
vs. unstimulated cells.
3.
Mix by shaking, then aseptically add alamarBlue in an amount equal to 10% of the volume in the well.
4.
Incubate cultures with alamarBlue for 4 - 8 hours. N.B. The optimum incubation time may vary between cell
types.
5.
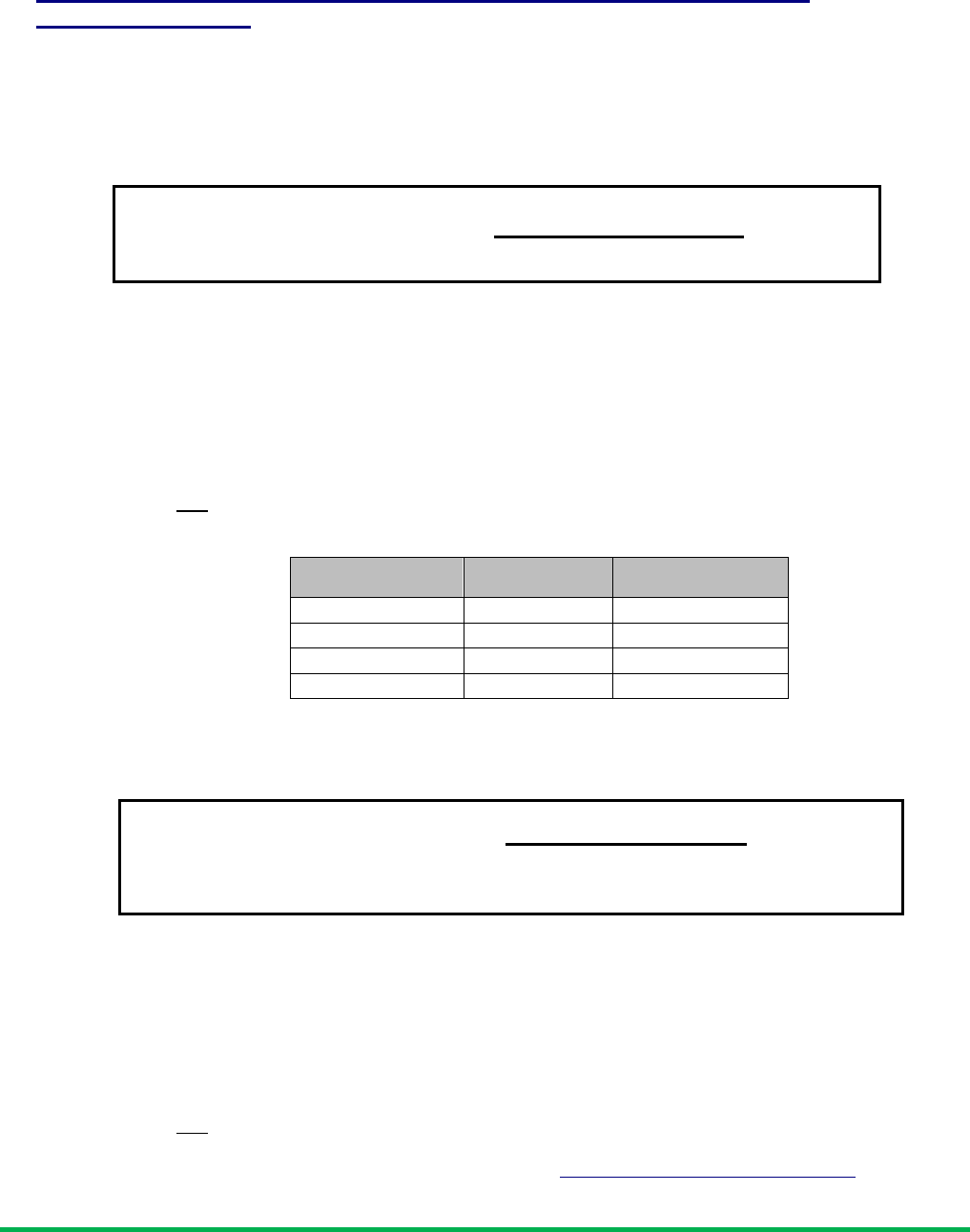
Measure cytotoxicity or proliferation using spectrophotometry of fluorescence.
TM
FI 590 of 100% reduced alamarBlue – Fl 590 untreated control
x 100
FI 590 of test agent – Fl 590 untreated control
Percentage
reduction of
(equation 2)
Page 6 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
Method for measuring cytotoxicity or proliferation using alamarBlue by
spectrophotometry.
6.
Measure absorbance at wavelengths of 570nm and 600nm after required incubation. Use a blank of media
only.
To calculate the percent difference in reduction between treated and control cells in cytotoxicity and proliferation
assays (Equation 3):
Percentage difference between
treated and control cells
(equation 3)
= (O2 x A1) - (O1 x A2) x 100
(O2 x P1) - (O1 x P2)
Where: O1 = molar extinction coefficient (E) of oxidized alamarBlue (Blue) at 570nm*
O2= E of oxidized alamarBlue at 600nm*
A1 = absorbance of test wells at 570nm
A2 = absorbance of test wells at 600nm
P1 = absorbance of positive growth control well
(cells plus alamarBlue but no test agent) at 570nm
P2 = absorbance of positive growth control well
(cells plus alamarBlue but no test agent) at 600nm
* Only one appropriate substitute wavelength may be used.
Wavelength
Reduced (R)
Oxidized (O)
540nm
104395
47619
570nm
155677
80586
600nm
14652
117216
630nm
5494
34798
Molar extinction coefficients for alamarBlue at different wavelengths
Alternatively, it may be useful to calculate the percent reduction of alamarBlue (Equation 1):
Where: O1 = molar extinction coefficient (E) of oxidized alamarBlue (Blue) at 570nm*
O2= E of oxidized alamarBlue at 600nm*
R1 = E of reduced alamarBlue (Red) at 570nm
R2= E of reduced alamarBlue at 600nm
A1 = absorbance of test wells at 570nm
A2 = absorbance of test wells at 600nm
N1 = absorbance of negative control well (media plus alamarBlue but no cells) at 570nm
N2 = absorbance of negative control well (media plus alamarBlue but no cells) at 600nm
* Only one appropriate substitute wavelength may be used.
For an Excel calculator and example calculations, please visit bio-rad-antibodies.com/alamarBlue.
(R1 x N2) - (R2 x N1)
x 100
(O2 x A1) - (O1 x A2)
=
Percentage reduction
of alamarBlue
(equation 1)

Page 7 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
General method for measuring cytotoxicity or proliferation using alamarBlue by
fluorescence.
6. Read fluorescence at excitation 560nm, emission 590nm.
To calculate percent difference in reduction between treated and control cells in cytotoxicity/ proliferation assays
use the following formula (Equation 4):
Where: FI 590 = Fluorescent Intensity at 590nm emission (560nm excitation).
If required, equation 2 can be used to find the percentage reduction of alamarBlue using fluorescence:
Where: FI 590 = Fluorescent Intensity at 590nm emission (560nm excitation).
For equation 2, it is necessary to include the fluorescence value for alamarBlue in its fully reduced form. To
produce the 100% reduced form of alamarBlue, simply autoclave a sample containing media and alamarBlue for
15 minutes. Since fluorescence units are arbitrary and may therefore vary depending upon instrument set up, it
is important that users determine the fluorescence reading for 100% reduction using the same media and
instrument as for their samples.
Worked calculations and Frequently Asked Question at bio-rad-antibodies.com/alamarBlue.
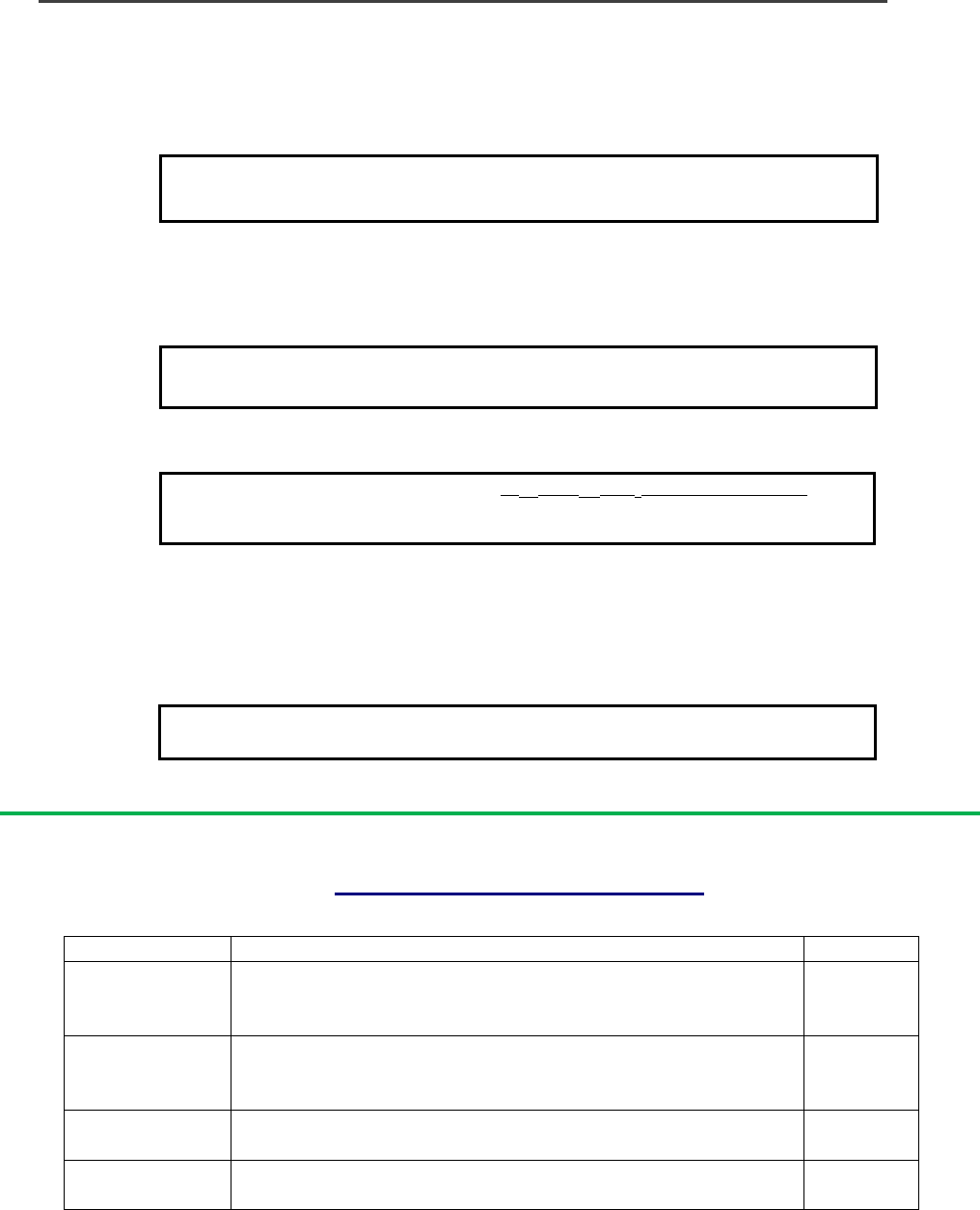
Method to determine LD50 using alamarBlue.
Use semi-log graph paper to plot the percent of untreated control (using equation 5) for each dilution of a given
test agent on the y-axis vs. the concentration of the test agent on the x-axis.
To determine the LD50 endpoint from the graph, read from where the 50 percent point intercepts the Dose
Response Curve to the concentration along the x-axis. This concentration is the LD50 value.
Determination of Doxorubicin LD50 using alamarBlue
FI 590 of test agent x 100
FI 590 of untreated control
Percentage difference
between treated and
control cells
(equation 4)
TM
FI 590 of 100% reduced alamarBlue – Fl 590 untreated control
x 100
FI 590 of test agent – Fl 590 untreated control
Percentage
reduction of
(equation 2)
% Growth Control
Page 8 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
Calculations for using alamarBlue in Spectrophotometry with different filters.
1.
Make up alamarBlue (AB) as directed in the package insert (1/10 dilution in 100μI media).
2.
Measure the absorbance of alamarBlue in media at the lower wavelength (LW) filter and at the higher
wavelength (HW) filter.
3.
Measure the absorbance of 100ul media only (blank) at the two wavelengths.
4.
Subtract the absorbance values of media only from the absorbance values of alamarBlue in media.
Where: AO
LW
= absorbance of oxidized form at the lower wavelength
AO
HW
= absorbance of oxidized form at the higher wavelength
5.
Calculate correction factor (R
0
):
6.
Calculate percentage difference in reduction between treated and control cells in cytotoxicity/proliferation
assays:
Where: A
LW
= absorbance at lower wavelength minus the media blank
A
HW
= absorbance at higher wavelength minus the media blank
R
0
= Correction factor (calculated in step 5)
7.
If the alternative calculation to find the percent reduction of alamarBlue is required, use the following
equation:
alamarBlue Appendix
Page
Appendix 1
Worked example to determine optimum cell density
and incubation time for A549 cells, using
alamarBlue and spectrophotometry.
9
Appendix 2
Worked example to determine optimum cell density
and incubation time for P388 cells, using
alamarBlue and spectrophotometry.
10
Appendix 3
Example alamarBlue reduction curves
for four cell lines using fluorescence.
11
Appendix 4
Effect of storage plates on
spectrophotometric readings.
11
Percentage difference in reduction = A
LW
– (A
HW
x R
0
) for test well x 100
(Equation 5) A
LW
– (A
HW
x R
0
) for control well
AO
LW
= Absorbance of AB in media – Absorbance of media only
AO
HW
= Absorbance of AB in media – Absorbance of media only
Correction factor for different filters:
R
0
= AO
LW
/AO
HW
Percentage reduction of alamarBlue = [ A
LW
- (A
HW
x R
0
) ] x 100
(Equation 6)

Page 9 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
Appendix 1: Worked example to determine optimum cell density and incubation time
for A549 cells, using alamarBlue and spectrophotometry.
The cell line A549 is a monolayer culture. The method given on page 4 was followed to find the percentage
reduction of alamarBlue after incubation with A549 cells at different densities and for different incubation periods.
Absorbance values at 570nm and 600nm after blanking with media only for A549 cells.
Absorbance values at 570nm
Absorbance values at 600nm
Time
Blue In
Media
500
cells/ml
1000
cells/ml
5000
cells/ml
10000
cells/ml
Blue In
Media
500
cells/ml
1000
cells/ml
5000
cells/ml
10000
cells/ml
(hours)
0
0.336
0.336
0.338
0.348
0.372
0.441
0.439
0.443
0.459
0.496
2
0.334
0.339
0.352
0.432
0.540
0.440
0.425
0.421
0.349
0.267
4
0.333
0.346
0.365
0.489
0.590
0.432
0.411
0.397
0.265
0.162
5.5
0.321
0.344
0.366
0.511
0.573
0.424
0.397
0.377
0.211
0.135
20
0.322
0.438
0.510
0.518
0.486
0.412
0.271
0.180
0.102
0.112
Using absorbance data from the sample data set, percent reduction of alamarBlue was calculated using
equation 1:
Percentage reduction of alamarBlue after incubation with A549 cells at different cell densities and
incubation periods.
Time
(hours)
Percentage reduction of alamarBlue
500
cells/ml
1000
cells/ml
5000
cells/ml
10000
cells/ml
0
6.3
6.1
6.0
5.7
2
8.6
11.5
35.4
65.7
4
11.9
17.3
57.7
89.9
5.5
13.6
20.4
70.0
91.8
20
49.6
76.2
88.3
80.7
These values are plotted to produce Figures 1a & 1b.
Figure 1a Figure 1b
Graphs showing percentage of alamarBlue reduction for A549 cells with different incubation periods
(Figure 1a) and at different initial cell densities (Figure 1b).
Please refer to the Worked Example section of bio-rad-antibodies.com/alamarBlue for notes to
accompany these graphs.
% Reduced
% Reduction

Page 10 of 11
Issue No: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
Appendix 2: Worked example to determine optimum cell density and incubation time for
P388 cells, using alamarBlue and spectrophotometry.
The cell line P388 is a suspension cell line. The method given on page 4 was followed to find the percentage
reduction of alamarBlue after incubation with P388 cells at different densities and for different incubation periods.
Absorbance values at 570nm and 600nm after blanking with media only for P388 cells.
Time
(hours)
Absorbance values at 570nm
Absorbance values at 600nm
Blue In
Media
500
cells/ml
1000
cells/ml
5000
cells/ml
10000
cells/ml
Blue In
Media
500
cells/ml
1000
cells/ml
5000
cells/ml
10000
cells/ml
0
0.336
0.342
0.334
0.332
0.328
0.441
0.451
0.438
0.433
0.422
2
0.334
0.340
0.333
0.335
0.335
0.440
0.448
0.435
0.422
0.404
4
0.333
0.339
0.332
0.339
0.346
0.432
0.444
0.432
0.414
0.391
5.5
0.321
0.331
0.325
0.335
0.344
0.424
0.435
0.424
0.404
0.377
20
0.322
0.332
0.328
0.381
0.434
0.412
0.423
0.415
0.337
0.253
Using absorbance data from the sample data set, percent reduction of alamarBlue was calculated using
equation 1:
Percentage reduction of alamarBlue for P388 cells at a range of cell densities following different
incubation periods
Time
(hours)
Percentage reduction of alamarBlue
500
cells/ml
1000
cells/ml
5000
cells/ml
10000
cells/ml
0
5.9
6.0
6.3
7.0
2
5.9
6.3
8.3
10.6
4
6.3
6.6
10.2
14.5
5.5
6.1
6.4
10.9
16.2
20
8.1
8.4
29.5
51.3
These values are plotted to produce Figures 2a & 2b.
Figure 2a Figure 2b
Graphs showing percentage of alamarBlue reduction for P388 cells with different incubation periods
(Figure 2a) and at different initial cell densities (Figure 2b).
Please refer to the Worked Example section of bio-rad-antibodies.com/alamarBlue for notes to accompany
these graphs.
60
50
40
30
20
10
5.5
20
500 cells/ml
1000 cells/ml
5000 cells/ml
10000 cells/ml
% Reduced
% Reduced

Page 11 of 11
Issue No.: 7
QIFUBUF012 alamarBlue Technical Datasheet IFU
Date: 16th August 2018
.
Appendix 3: Example alamarBlue reduction curves for four cell lines
using fluorescence.
Examples of reduction curves are included to demonstrate the usefulness of the alamarBlue assay for
measuring cell proliferation.
Detection of cell growth of four cell lines using alamarBlue.
Appendix 4: Effect of storage plates on spectrophotometric readings.
The absorbance readings of alamarBlue may be affected by the type of plates which are used for experiments. A
series of absorbance readings were taken for the oxidized and reduced forms of alamarBlue using a range of
different types of plates. Following the readings on day 1, plates were covered in foil and refrigerated prior to
further readings on days 2 & 3. See Frequently Asked Question 11 at bio-rad-antibodies.com/alamarBlue for
more information about storing plates prior to collecting data.
Plate
Type
Day
Absorbance
BLUE (Oxidized)
RED (Reduced)
540nm
570nm
600nm
630nm
540nm
570nm
600nm
630nm
Dynatech
Flat
Bottom
Day 1
(0.003)
(0.005)
(0.007)
(0.003)
(0.020)
(0.027)
(0.002)
(0.000)
0.298
0.496
0.708
0.236
0.693
1.017
0.126
0.075
Day 2
(0.003)
(0.004)
(0.006)
(0.002)
(0.020)
(0.027)
(0.008)
(0.009)
0.294
0.484
0.692
0.227
0.697
1.018
0.164
0.118
Day 3
(0.003)
(0.006)
(0.008)
(0.003)
(0.010)
(0.024)
(0.008)
(0.009)
0.296
0.486
0.691
0.231
0.734
1.038
0.199
0.149
Corning
Flat
Bottom
Day 1
(0.002)
(0.004)
(0.005)
(0.002)
(0.020)
(0.024)
(0.003)
(0.004)
0.210
0.335
0.474
0.169
0.530
0.772
0.137
0.105
Day 2
(0.002)
(0.003)
(0.004)
(0.001)
(0.020)
(0.027)
(0.004)
(0.004)
0.210
0.329
0.458
0.161
0.580
0.822
0.193
0.159
Day 3
(0.002)
(0.003)
(0.005)
(0.002)
(0.020)
(0.035)
(0.004)
(0.003)
0.200
0.322
0.444
0.160
0.600
0.823
0.210
0.172
Corning
Round
Bottom
Day 1
(0.001)
(0.002)
(0.002)
(0.001)
(0.014)
(0.018)
(0.002)
(0.000)
0.380
0.635
0.913
0.300
0.870
1.266
0.151
0.084
Day 2
(0.002)
(0.003)
(0.004)
(0.002)
(0.011)
(0.014)
(0.002)
(0.002)
0.390
0.641
0.914
0.295
0.850
1.241
0.146
0.083
Day 3
(0.004)
(0.006)
(0.008)
(0.004)
(0.017)
(0.021)
(0.007)
(0.006)
0.390
0.646
0.916
0.302
0.860
1.237
0.159
0.094
Absorbance of alamarBlue oxidized and reduced forms for a range of plates, including those refrigerated
prior to data collection. 100ul of RPMI 1640 with MOPS pH7.0, no phenol red. Standard deviations are in
parentheses (calculated for n=8).
100 156 312 625 1250 2500 5000 10000
1000
0
HT1080
6000
5000
4000
3000
2000
Fluorescence
