
EB1895
SOIL MANAGEMENT FOR
SMALL FARMS
This publication is part of the Farming West of the Cascades series
Craig Cogger
What Is Soil?
Soil is a natural mixture of weathered rock fragments and organic matter
that has formed at the surface of the earth. It is biologically active—a home
to countless microorganisms, invertebrates, and plant roots. It varies in depth
from a few inches to five or more feet. Soil is roughly 50% pore space, a
complex network of pores of varying sizes, much like those in a sponge.
Soil provides nutrients, water, and physical support for plants, and air for
plant roots. Soil organisms are nature’s prime recyclers, turning dead cells
and tissue into nutrients, energy, carbon dioxide, and water to fuel new life.
Soil and Water
Soil Pores, Water, and Productivity
A productive soil can take in and hold water and supply water to plants.
A soil’s permeability and water holding capacity depend on its network
of pores.
• Large pores, or macropores, control the permeability and aeration of a
soil. Macropores include earthworm channels and many root channels.
They are large enough that water moves through them rapidly by gravity,
allowing rainfall and irrigation water to infiltrate into the soil and
excess water to drain through the soil.
• Micropores are fine soil pores, typically a fraction of a millimeter in
diameter. They are responsible for the water holding capacity of soil.
Micropores hold water by capillary forces, like the fine pores in a
sponge or towel. Much of the water held in micropores is available
to plants, while some is held so tightly that plant roots cannot tap it.
Soil that has a balance of macropores and micropores will provide adequate
permeability and water holding capacity for good plant growth. Soils that
contain mostly macropores will take in water readily, but they will not hold

2 — Soil Management for Small Farms
much water. As a result, they need more frequent
irrigation. Soils that contain mostly micropores will
have good water holding capacity, but they will take
longer to dry and warm in the spring. They do not
take in water readily, thus rainfall and irrigation
water may run off the soil surface.
What Affects the Porosity of Your Soil?
A number of soil properties affect the abundance of
macropores and micropores. These include texture,
structure, compaction, and organic matter. You can
evaluate these properties to understand how they
affect the porosity of your soil. The only tools you
need are your eyes, your fingers, and a shovel.
Soil texture. Texture describes how coarse or fine
a soil is; it depends on the relative amounts of sand,
silt, and clay particles in the soil. The coarsest soil
particles are sand. They are visible to the eye, and
they give soil a gritty feel. Silt particles are smaller
than sand, about the size of individual particles of
white flour. They give soil a smooth, floury feel.
Sand and silt particles look like miniature rocks.
Clay particles are the finest, similar in size to tiny
bacteria and viruses, and they typically have a flat
shape. Soils rich in clay feel very hard when dry,
but they are easily shaped and molded when moist.
Although all of these particles seem small, the rel-
ative difference in their sizes is quite large. If a typical
clay particle were the size of a penny, a sand particle
would be as large as a house.
Soil texture affects porosity. Pores between sand
particles tend to be large, while pores between silt
and clay particles tend to be small. Thus, sandy soils
contain mostly macropores, promoting permeability
but limiting water holding capacity. Clayey soils
contain mostly micropores, creating high water
holding capacity but reducing permeability.
Particle size also affects the surface area in a volume
of soil. Surface area is important because surfaces are
the most active part of the soil, holding plant nutri-
ents, binding contaminants, and providing a home for
microorganisms. Clay particles have a large surface
area relative to their volume; a small amount of clay
makes a large contribution to the surface area of a
soil.
Nearly all soils contain a mixture of particle sizes
and a pore network of varying pore sizes. A soil that
has roughly equal influence of sand, silt, and clay
particles is called a loam. Loams usually make good
agricultural and garden soils because they have a
balance between macropores and micropores. Loams
usually have good water holding capacity and moder-
ate permeability.
A sandy loam is similar to a loam, except that it
contains more sand. It feels gritty, yet has enough silt
and clay to hold together in your hand. Sandy loams
usually have low to moderate water holding capacity
and good permeability. Silt loams are richer in silt,
and feel smooth rather than gritty. They are pliable
when moist, but they are not very sticky. Silt loams
usually have high water holding capacity and low
to moderate permeability. Clays and clay loams are
very hard when dry, and sticky when wet. They can
be molded into wires and ribbons when moist. They
generally have high water holding capacity and low
permeability.
Many soils contain coarse fragments—gravel and
rocks. Coarse fragments do not contribute to the
productivity of a soil, and they can be a nuisance
when tilling. Most agricultural soils have less than
15% coarse fragments in the plow layer.
Soils with many different textures can be suitable for
farming, as long as you are aware of the soil’s limita-
tions and use appropriate management. Sandy soils
need lighter, more frequent irrigation and fertilization,
but you can till them earlier in the spring. Clay soils
hold more water, but they are harder to till, and they
dry more slowly in the spring.
Soil structure. Individual particles of sand, silt, and
clay tend to cluster and bind together in soil, forming
aggregates called peds. Aggregation is a natural
process in soil, caused largely by biological activity,
Table 1. Soil particle sizes.
Particle Diameter
Sand 0.05—2 mm
Silt 0.002—0.05 mm
Clay <0.002 mm
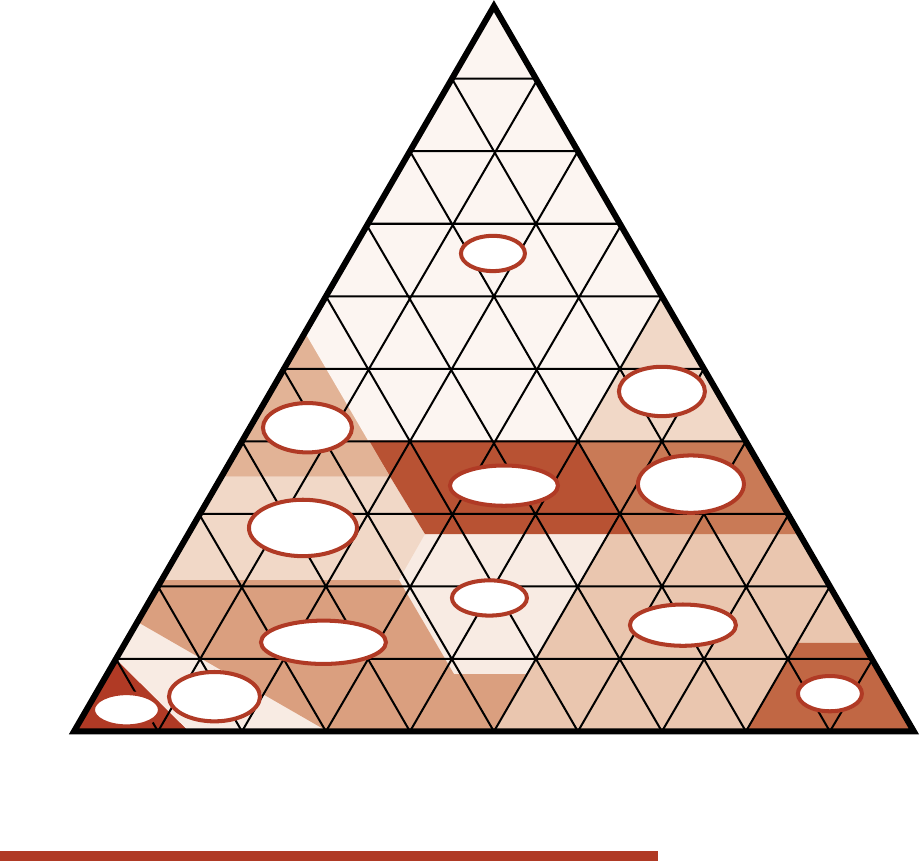
Soil Management for Small Farms — 3
100
1090
2080
70 30
60 40
50 50
6040
30 70
8020
10 90
100
100
90 80 70 60 50 40 30 20 10
Percent Clay
Percent Silt
Percent Sand
Clay
Silty
clay
Silty clay
loam
Clay loam
Sandy
clay
Sandy
clay loam
Sandy loam
Loamy
sand
Sand
Loam
Silt loam
Silt
including earthworm burrowing, root growth, and
microbial action. Soil organic matter is an important
binding agent that stabilizes and strengthens the peds,
providing structure to the soil. Dig up a piece of grass
sod and examine the soil around the roots. The
granules of soil you see hanging onto the grass roots
are examples of peds—containing sand, silt, clay,
and organic matter.
In medium- to fine-textured soils, good structure is
important because it increases the macroporosity of
the soil. The spaces between peds are macropores,
improving permeability, drainage, and recharge of
air into the soil profile. The pores within peds are
predominantly micropores, contributing to the
water holding capacity of the soil.
Compaction and loss of structure. Soil
structure is fragile and can be damaged
or destroyed by compaction, exces-
sive tilling, or tilling when the soil
is too wet. Loss of organic matter
also weakens structure. Compaction alters the struc-
ture of the soil, squeezing macropores into micro-
pores and creating horizontal aggregates that resist
root penetration and water flow. You can protect the
structure of your soil by avoiding unnecessary traffic
on the soil, and by postponing tillage until the soil has
become dry enough to till. If you can mold a piece of
soil into a wire or worm in your hand, it is too wet for
tilling. If the soil crumbles when you try to mold it, it
is dry enough to till.
Sometimes a compacted layer or “plow pan” forms
just below the depth of tillage. Occasionally, tilling
deeper helps break up a plow pan.
Organic matter. Adding organic matter is the
best way to improve the plant environment in
nearly all soils. Organic matter helps build
and stabilize soil structure in fine-textured
and compacted soils, improving soil perm-
eability and aeration, and reducing the
risk of runoff and erosion. The
Percentages of clay, silt,
and sand in the basic
soil textural classes.

biological decomposition of organic materials pro-
duces natural glues, which bind and strengthen soil
aggregates. Organic matter also helps sandy soils
hold water and nutrients. Refer to pages 20–22 for
information on amending soil with organic matter.
Effect of Porosity on Irrigation
Most areas in the Northwest require summer irriga-
tion for peak crop production. Irrigation is essential
on sandy soils. The need for irrigation varies, depend-
ing on soil water holding capacity, weather, site aspect,
and crop requirements. In most cases, the goal of
irrigation is to recharge the available water in the top
foot or so of the soil. For a sand, one inch of irriga-
tion water will recharge the water holding capacity.
Any more will leach through the root zone, carrying
nutrients with it. A silt loam or clay can hold more
than 2 inches of water, but you may need to irrigate
more slowly than for a sandy soil to avoid runoff.
Site and Landscape Factors
Landscape Position
Landscape position affects the suitability of a site
for the production of specific crops. Ridge tops and
sideslopes tend to shed water, and soils in these land-
scape positions are likely to be droughty and subject
to erosion. Soils at the bottom of slopes and in low
areas collect water, and are likely to be wet late into
the spring. Soils on level ground can also be wet
during the winter and spring, especially if they have
a fine-textured or compacted subsurface layer that
restricts the downward movement of water.
Wet Soils
If your soil stays wet in the spring, you will have to
delay working the soil and planting. Working wet soil
can damage the structure, and planting in cold, wet
soil reduces germination. Some plants don’t perform
well in wet soils. Raspberries, for example, become
infected by a root disease in wet soils, lose vigor and
may die.
Soil color gives clues to the wetness of a soil. If the
subsoil is a brown or reddish color, the soil is usually
well drained with few wetness problems. Gray and
mottled subsoils are often saturated during the wet
season.
If you have wet areas on your farmland, avoid the
temptation to till too early in the season, and avoid
crops that are sensitive to wet conditions. Mid-season
annual crops such as sweet corn, green beans, and
squash, and some perennial forages are good choices
for wet soils. Blueberries can be a suitable crop on
moderately wet soils.
Some farmland has subsurface drainage or ditches
that lower the water table and help the soil dry more
quickly. If your land has drainage you can maintain
and repair it to keep it functioning well. If your land
is wet and undrained, you may not be able to install
drainage because of wetland regulations. If you have
questions about field drainage, check with your local
Natural Resources Conservation Service (NRCS)
office. You can find it in the government pages of
the phone book listed under Federal Government,
Department of Agriculture.
Raised beds can improve drainage in marginal situa-
tions. The simplest raised beds involve hilling soil
in rows during tillage. Raspberry growers frequently
hill soil around raspberry plants because the raspberry
roots will grow into the more aerated soil in the hilled
area, reducing problems with root rot. More sophisti-
cated raised beds can be quite expensive, and usually
they are more suitable for gardens than farms. Raised
beds may be economical for farmers in some cases;
for farmers growing a small area of high value crops
under intensive management, installing raised beds
may make sense.
Runoff and Erosion
Runoff and soil erosion can be a serious problem on
sloping ground. Erosion affects soil quality and crop
productivity by reducing the depth of topsoil. Runoff
and erosion can also affect water quality when eroded
soil or dissolved contaminants run off into surface
water. If you are farming sloping ground, follow
recommended conservation practices to reduce run-
off and erosion. These practices include minimum
tillage, cover cropping, contour planting, and strip
rotations. The key to these practices is keeping
vegetative cover or crop residues on the surface as
4 — Soil Management for Small Farms

much as possible to help water soak into the soil
rather than run off. Check with your local NRCS
office for information on conservation practices
that are appropriate for your farm.
Site Aspect
Site aspect has an important effect on crop growth.
South- and southwest-facing exposures collect the
most sunlight and heat and use the most water. North-
and northeast-facing exposures are cooler and retain
more water. Low-lying areas can be prone to early
and late frosts. South and southwest exposures are
a good location for crops grown for early-season
markets and crops that need a lot of heat units to
ripen. Always consider site aspect when looking to
lease or purchase land, or when planning for crop
production.
Soil Horizons and Depth
Soil typically has several layers or horizons that were
formed by natural weathering processes. Sometimes
different layers formed during different geological
events.
The surface soil, or topsoil, is the darkest color and
contains the most organic matter. It is the most bio-
logically active layer and contains the largest proportion
of available nutrients. Topsoils in western Washington
range in depth from about 3 inches to 12 inches.
The subsoil contains less organic matter than the
topsoil, and it is lighter in color. Its texture can be
coarser, finer, or similar to the topsoil. The subsoil
provides additional water and nutrients to crops.
Deep subsoils with moderate to high water holding
capacity greatly increase the ability of deep-rooted
crops to survive drought. Well-drained subsoils have
uniform brown or reddish colors, while wet subsoils
are usually gray, flecked with bright-colored mottles.
Beneath the subsoil is relatively unweathered material
called parent material. The parent material in most
soils contains few roots and little or no structure.
Biological activity is much lower than in the topsoil
or subsoil.
Some soils have layers that restrict root growth. In
western Washington, the most common restrictive
layers are compact “hardpans” in glacial soils, or
coarse gravelly layers that hold little water. Other
restrictive layers include tight clay horizons and
shallow bedrock. Soils with shallow root zones will
have less available water and fewer nutrients than a
similar soil with a deeper root zone.
Soils of Western Washington
Most of the agricultural soils in western Washington
are in the lowlands, below 1,200 feet elevation. This
section describes the major types of lowland soils in
western Washington, how they were formed, and
their suitability for agriculture.
Alluvial Soils
The alluvial soils in the major river valleys through-
out western Washington are by far the best farmlands
in the area. These soils were formed by repeated
flooding cycles that have occurred since the most
recent glacial retreat about 15,000 years ago. The
valley soils are deep, level, and nearly free from
rocks. Most are sandy loam to silt loam in texture.
They have good to excellent water holding capacity,
good nutrient holding capacity, and low erosion
potential. They are easy to till with light equipment
and suitable for a variety of crops. Some areas are
wet late into the spring and are not suitable for early
crops or crops sensitive to wet soils. Other areas are
well drained. Most of the alluvial soils in King and
Pierce counties have been lost to development, and
development encroaches on alluvial farmland in
other counties as well.
Glacial Soils
Most of the other soils in the Puget Sound area formed
from glacial materials on low plateaus. The glacial
soils developed from three main types of glacial
material: till, outwash, and lacustrine (lakebed)
deposits.
Glacial till is material left behind by glacial ice. Soils
developed from till typically have a sandy loam to
loam texture, containing more than 15% gravel and
rocks. These soils are usually 18 to 36 inches deep
and are underlain by a “hardpan” that consists of very
dense and cemented till that was compacted by the
Soil Management for Small Farms — 5

weight of the glacial ice sheet. The dense layer
restricts root growth and water movement, and it is
too thick and too compact to break up. Glacial till
soils have a moderate water holding capacity. They
are frequently sloping and somewhat rocky, and low
areas tend to be wet. Organic matter levels are gener-
ally low. Despite these limitations they are suitable
for pastures and moderately productive for row crops.
Organic matter, conservation tillage, and careful water
and nutrient management will make these soils more
productive.
Glacial outwash was deposited by glacial melt-
water streams. Outwash soils are found throughout
the Puget Sound area and in some parts of southwest
Washington. Outwash soils are usually coarse tex-
tured—sandy or gravelly. In Whatcom County, the
outwash has a cap of silty material about a foot thick
that was deposited by wind. Some sandy outwash
soils are moderately productive and are good soils
for early crops and crops needing well-drained con-
ditions. Careful irrigation management and nutrient
management are essential to successful crop produc-
tion. Gravelly outwash soils are too droughty for
farming. Outwash soils with a silty cap hold more
water than other outwash soils, and they naturally
contain higher levels of organic matter. They vary
in drainage, and are suitable for a variety of crops.
Lacustrine soils formed in material deposited at the
bottom of ancient glacial lakes. They typically have a
silt loam texture in the surface horizon, and silt loam
to clay loam texture in the subsoil. They have a high
water holding capacity and can be productive under
good management. Limitations include wetness late
into spring, and risk of runoff and erosion on sloping
ground. They can be hard when dry.
Volcanic Soils
Areas of eastern King and Pierce counties have soils
developed from volcanic mudflow materials. These
soils are level with a black, loamy topsoil and a dense,
rocky subsoil. Most mudflow soils have restricted
drainage, and are wet during the winter and spring.
They are well suited to pastures and acceptable for
mid-season row crops. They are too wet for early
crops or crops that require good drainage.
Volcanic ash and sediments dominate some soils in
Lewis and Cowlitz counties. Suitability for farming
varies, depending on slope and texture.
Weathered Soils of Southwest Washington
Most areas south of Olympia were not covered by
glacial ice 15,000 years ago, and the soils are quite
different from soils in the Puget Sound area. They
tend to be older, more weathered, and higher in clay
content than the glacial soils. They generally have
fewer coarse fragments and a more stable structure.
They formed in sediments from old terraces, ancient
glacial material, and upland material. Most of these
soils range in texture from loam to clay, and are found
on a variety of slopes. Gently sloping soils on well-
drained landscapes are productive agricultural soils
when good conservation practices are used. Maintain-
ing organic matter and soil structure are essential in
the finer textured soils. Wetter and more sloping soils
are better suited for pasture than row crops.
Evaluating Soils
Evaluating the soil is an important part of choosing
farmland. If you are planning to buy or lease farm-
land, learn as much about the soils as you can, keep-
ing the following in mind: soil texture, structure,
compaction, depth, drainage and wetness, landscape
position, and site aspect. All these will affect site
productivity and suitability for different crops. Don’t
limit your investigation to the topsoil. Dig or probe
to a depth of three feet in a few spots to determine
the depth and properties of the underlying soil.
Soil surveys are a tool you can use to identify poten-
tial farmland or learn more about land you already
lease or own. Each county has a soil survey that
6 — Soil Management for Small Farms

contains maps showing locations of different soil types
and descriptions of each soil type. Because each soil
type can have a range of properties and because several
soil types often are mixed together on the landscape,
the soil survey map does not necessarily match what
you find on a piece of land. Use the survey as a guide
for understanding soils in an area, but walk and dig
on a piece of land to confirm what the soil is like there.
You can request a copy of a soil survey at your local
NRCS office. Some surveys are out of print, but you
can visit the NRCS office to look at one of their
copies.
Soil Organisms
Soil abounds with life. Besides plant roots, earthworms,
insects, and other creatures that we can see, soil is
home to an abundant and diverse population of micro-
organisms. A single gram of topsoil (about one quarter
of a teaspoon) may contain a billion microorganisms
(Table 2). Microorganisms are most abundant in the
rhizosphere—the thin layer of soil surrounding plant
roots.
The main function of soil organisms is to decompose
the remains of plants and other organisms, releasing
energy, nutrients, and carbon dioxide, and creating
soil organic matter. Organisms at all levels, from tiny
bacteria to insects and earthworms, take part in this
food web. Mammals such as moles and voles are
also part of the food web, feeding on insects and
earthworms.
Some soil organisms play other beneficial roles as well.
Mycorrhizae are fungi that infect plant roots and
increase the roots’ ability to take up nutrients from
the soil. Rhizobia bacteria are responsible for nitrogen
fixation. Earthworms mix large volumes of soil and
create macropore channels that improve permeability
and aeration.
Not all soil organisms are beneficial to agriculture.
Some are pathogens, causing a variety of diseases,
such as root rot of raspberries and scab on potatoes.
The activity of soil organisms depends on soil mois-
ture and temperature. Microorganisms are most active
between 70° and 100°F, while earthworms are most
active and abundant at about 50°F. Most organisms
prefer moist soil. Because organic matter is at the
base of the soil food web, soils with more organic
matter tend to have more organisms. Just about any
activity affects the population and diversity of soil
organisms—including tillage, the use of fertilizers,
manures, and pesticides, and choice of crop rotations.
The relationships between farming practices, microbial
populations, and soil quality are complex and often
poorly understood. Amending soils with organic matter,
returning crop residues to the soil, and rotating plantings
are practices that tend to increase the number and
diversity of beneficial organisms.
Nutrient Management, Fertilizers,
and Manures
Soil supplies 13 essential plant nutrients (Table 3).
Each nutrient plays one or more specific roles in the
function of the plant. Nitrogen, for example, is part
of the structure of molecules of chlorophyll, amino
acids, proteins, DNA, and many plant hormones. It
plays a vital role in nearly all aspects of the growth
and development of the plant, and plants need large
amounts of nitrogen to grow well. By contrast, molyb-
denum is involved in the function of a few enzymes,
and plants need only tiny amounts. Molybdenum is
nonetheless essential, and plants do not grow well
in a soil that is deficient in molybdenum.
Nutrients are classified as primary nutrients, secondary
nutrients, and micronutrients, based on the amounts
of them plants need (Table 3). When the soil nutrient
Soil Management for Small Farms — 7
Table 2. Approximate abundance of
microorganisms in agricultural topsoil.
Organism Number per gram
(dry weight basis)
Bacteria 100 million to 1 billion
Actinomycetes 10 million to 100 million
Fungi 100 thousand to 1 million
Algae 10 thousand to 100 thousand
Protozoa 10 thousand to 100 thousand
Nematodes 10 to 100

supply is deficient, farmers use fertilizers to provide
the additional nutrients needed for healthy plant
growth.
Nutrient Deficiencies
The most common nutrient deficiencies are for the
primary nutrients, N, P, and K. Nearly all agricultural
soils lack enough available N for ideal plant growth.
Sulfur deficiencies are common in western Washing-
ton, and calcium and magnesium may be deficient in
acid soils. Except for boron and zinc, growers in this
region seldom encounter micronutrient deficiencies.
Boron deficiencies occur in western Washington,
particularly in root crops, brassica crops, and cane-
berries, such as raspberry. Zinc deficiency is most
often associated with high pH soils, especially on
tree fruit. Plants with nutrient deficiencies sometimes
have characteristic symptoms; they also grow more
slowly, yield less, and are less healthy than plants
with adequate levels of nutrients.
Excess Nutrients
Excess nutrients can be a problem for plants and the
environment. Excesses usually result from applying
too much of a nutrient, or applying it at the wrong
time. Too much boron is toxic to plants. Too much
nitrogen can lead to excessive production of foliage
(increasing the risk of disease and wind damage),
delayed flowering and fruiting, and delayed dormancy.
Extra available nitrogen left in the soil at the end
of the growing season can leach into groundwater,
degrading the quality of drinking water. Excess
levels of nitrogen most often occur on soils where
large amounts of manure have been used. The key
8 — Soil Management for Small Farms
Table 3. Essential plant nutrients. Plants obtain these elements from soil,
fertilizers, crop residues, and other amendments. Plants also require
carbon, hydrogen, and oxygen, which they derive from water and air.
Name Chemical Plant-Available Solubility
Symbol Ions in Soil Water
Primary Nutrients
Nitrogen N NH
4
+
, NO
3
-
,NO
2
-
high
Phosphorus P HPO
4
=
, H
2
PO
4
-
very low
Potassium K K
+
low
Secondary Nutrients
Sulfur S SO
4
=
high
Calcium Ca Ca
++
low
Magnesium Mg Mg
++
low
Micronutrients
Zinc Zn Zn
++
very low
Iron Fe Fe
++
, Fe
+++
very low
Copper Cu Cu
++
, Cu
+
very low
Manganese Mn Mn
++
, Mn
++++
very low
Boron B H
3
BO
3
medium
Molybdenum Mo MoO
4
=
low
Chlorine Cl Cl
-
high
to applying fertilizers and manures is to meet plant
nutrient needs without creating excesses that can
harm plants or the environment.
How Nutrients Become Available to Plants
Plants can only take up nutrients that are dissolved in
soil water (in solution). Most nutrients in soil are not
in solution; they exist in the soil minerals and organic
matter in insoluble forms.
Soil nutrients become available to plants only after
they dissolve into soil solution. This occurs by weather-
ing of mineral matter and biological decomposition
of organic matter. Weathering of mineral matter is a
very slow process, releasing small amounts of nutri-
ents each year. The rate of nutrient release from soil
organic matter is somewhat faster, depending on the
amount of biological activity in the soil. Nutrient
release is fastest when soils are warm and moist, but
it is nearly zero when soils are cold or dry. About 1
to 4% of the nutrients in soil organic matter are
released in soluble form each year.
Soluble, available nutrients are in ionic form. An ion
has either positive or negative charges. Positively-
charged ions are cations, and negatively charged ions
are anions. Clay particles and soil organic matter have
negative charges on their surfaces, and they can attract
cations (such as potassium, calcium, and magnesium).
The clay and organic matter surfaces hold nutrient
cations in a ready reserve form that can be released
rapidly into soil solution to replace nutrients taken up by
plant roots. This reserve supply of nutrients contributes
to the fertility of a soil. The capacity of a soil to hold
cations is called its cation exchange capacity, or CEC.
Nitrogen and Phosphorus
Nitrogen and phosphorus present the greatest nutrient
management challenges on most farms. Plants need
large amounts of both nutrients, but excess levels of
either nutrient increase the risk of water quality prob-
lems. Understanding the availability and cycling of
these nutrients can help growers become better
nutrient managers.
The nitrogen cycle. Most nitrogen in soil is in the
organic matter in forms such as humus and proteins.
This organic nitrogen is not available to plants. As
the soil warms in the spring, soil microbes begin
Soil Management for Small Farms — 9
Most nutrients are in in-
soluble form, as part of the
structure of the soil mineral
matter and organic matter.
Weathering of mineral matter and
biological decomposition of organic
matter slowly release nutrients into
soluble, available forms.
Ca
Mg
K
Ca
++
Mg
++
K
+
Ca
++
Mg
++
K
+
- -
- -
-
Plant roots take up
available nutrients
from the soil solution.
Clay and organic
matter surfaces act
as a ready reserve
supply of cationic
(+ charged) nutrients.
These nutrients are
released rapidly to
the soil solution by
cation exchange.
Table 4. Common forms of nitrogen in soil.
Organic N
Main form in soil. Found in organic matter in
forms such as proteins, lignin, amino acids,
and humus. Not available to plants. Mineral-
ized to ammonium by soil microorganisms.
Ammonium N (NH
4
+
)
Soluble form. Available to plants. Converted
to nitrate by soil microorganisms.
Nitrate N (NO
3
-
)
Soluble form. Available to plants. Can be lost
by leaching. Converted to gases in wet soils.
Atmospheric N (N
2
)
Comprises about 80% of soil atmosphere.
Source of N for N-fixing plants. Not used by
other plants.
Unavailable and available forms of plant
nutrients.
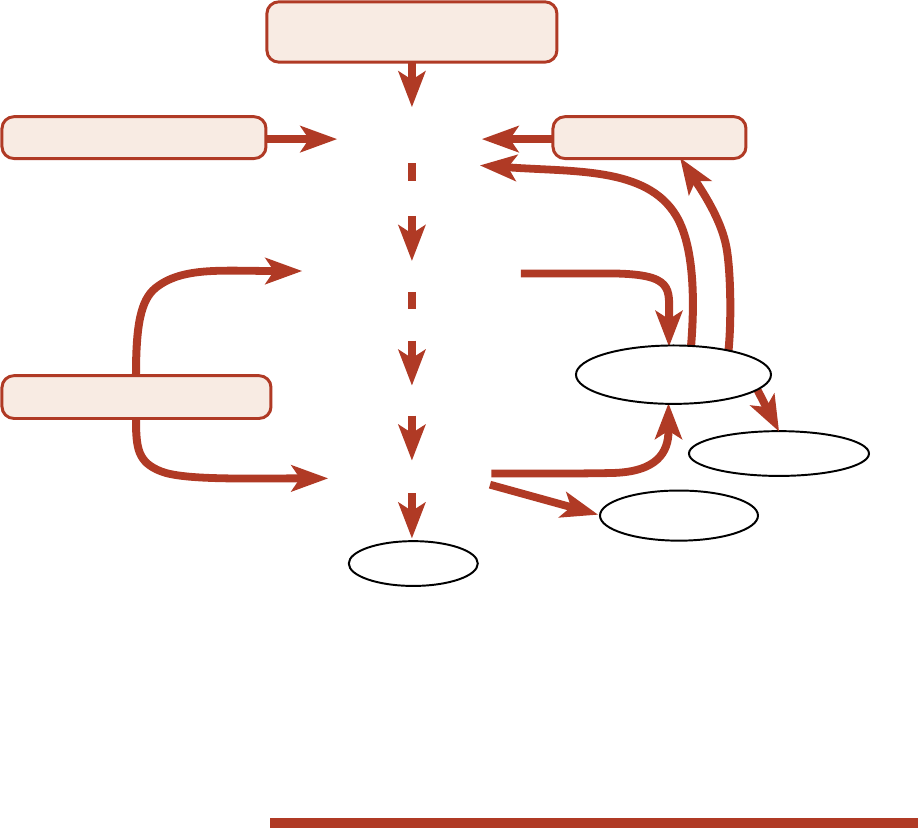
to decompose organic matter, releasing some of the
nitrogen as ammonium (NH
4
+
). Ammonium is a soluble
ion that is available to plants and soil microbes. When
the soil is warm, a group of microbes called nitrifiers
convert the ammonium to nitrate (NO
3
-
). Nitrate is
also soluble and available to plants. The ammonium
and nitrate ions released from soil organic matter
are the same as ammonium or nitrate contained in
processed fertilizers.
Because nitrate has a negative charge, it is not held
to the surfaces of clay or organic matter, and it can
be lost readily by leaching. Nitrate remaining in the
soil at the end of the growing season will leach during
the fall and winter, and it may leach to groundwater
where it becomes a contaminant. In soils that become
saturated during the wet season, soil microbes convert
nitrate to nitrogen gases, which diffuse back into the
atmosphere.
Nitrogen cycle: (a) Legumes, soil organic matter, crop residues and organic additions (manures, composts, etc.) are
sources of organic N. (b) Organic N is mineralized into ammonium (NH
4
+
) by soil microbes. (c) Commercial fertilizer
supplies N as ammonium or nitrate (NO
3
-
). (d) Microbes nitrify ammonium to nitrite and then nitrate. (e) Plants,
microorganisms, leaching below the root zone, and release of gaseous N to the atmosphere remove N from the
root zone soil solution. (f) Crop harvest removes N stored in plants. (g) Nitrogen present in both crop residues and
soil microorganisms becomes a part of the soil organic N content.
Organic Additions
(manure, compost, etc.)
Soil Organic
Nitrogen
Crop Residues
N-fixation (legumes)
Mineralization (microbes)
NH
4
+
(ammonium)
Nitrification (microbes)
NO
2
-
(nitrite)
NO
3
-
(nitrate)
Leaching
Harvest removal
Gaseous
nitrogen loss
Commercial Fertilizer
(a)
(a)
(a)
(b)
(c)
(c)
(d)
(e)
(e)
(e)
(e)
(f)
(g)
Plants and soil
microorganisms
Ammonium and nitrate taken up by plants become
organic forms in the plant tissue. When plant residues
are returned to the soil, they decompose, slowly
releasing nitrogen into available forms and complet-
ing the cycle.
The nitrogen cycle is a leaky one, with losses to leach-
ing and to the atmosphere. Harvesting crops removes
more nitrogen. To maintain an adequate nitrogen
supply, nitrogen must be added back into the system
through fixation or fertilization. Nitrogen fixation is
a natural process involving certain plants and Rhizo-
bia bacteria. The Rhizobia form nodules in the plant
roots; through these nodules they are able to supply
atmospheric nitrogen (N
2
) from the soil air to the host
plant. Legumes such as peas, beans, alfalfa, clover,
and scotch broom are common nitrogen-fixers. Alder
trees also fix nitrogen. When tissue from N-fixing
plants decomposes, the fixed nitrogen becomes
10 — Soil Management for Small Farms

available to other plants. Farmers use legumes as cover
crops or in crop rotations to supply nitrogen to future
crops. About one half of the nitrogen in a legume cover
crop will become available to the following crop.
Buying feed to raise animals brings nitrogen onto
the farm in the feed. The animals cycle a portion of
the feed nitrogen to the soil through their manure.
Manure is a good source of nitrogen for crops and
pastures, but adding excessive amounts of manure
leads to over-fertilization, increasing the risk of harm
to crops and water quality. For more information on
using manure, see pages 13–16 and Fertilizing with
Manure, PNW0533, part of the Farming West of the
Cascades series available from Washington State
University Cooperative Extension.
Phosphorus. Available forms of phosphorus are
released from the mineral and organic fractions of
the soil through the weathering and decomposition
processes described on page 9. Unlike nitrogen, the
available forms of phosphorus have limited solubil-
ity, and they revert to insoluble forms in the soil.
In the spring, when soils are still cool, organic matter
decomposition is slow, and little phosphorus is avail-
able for plants. It is especially difficult for seedlings
or transplants to obtain phosphorus early in the season
because their limited root system compounds the effect
of low availability. The plants often have a purplish
tinge associated with phosphorus deficiency. Many
crops respond to phosphorus-rich starter fertilizer
placed near the seed or transplants to help overcome
early deficiencies. Most plants outgrow the deficien-
cies as the season continues, because phosphorus
availability increases in warmer soils, and root systems
grow larger and become more able to tap available
phosphorus.
Phosphorus levels can be quite high in soils with a
history of manure application, although you may
still see some signs of early season phosphorus
deficiency in these soils. The risk of water quality
problems from excess phosphorus is higher in soils
with high phosphorus levels. Phosphorus can be a
problem in surface water, where it can lead to the
excessive growth of aquatic plants. In the Northwest,
lakes are usually the most sensitive to phosphorus.
Phosphorus can enter surface water in runoff, in
eroded sediments, or through shallow groundwater.
The environmental risk depends on the capacity of the
soil to hold phosphorus in unavailable forms, the amount
of phosphorus added to the soil, the amount of runoff
and erosion, and the sensitivity of surface water to
phosphorus.
Understanding Fertilizers
Fertilizers supplement the native nutrient supply of the
soil. They are essential to good plant growth when soil
nutrient supply is inadequate. You can use processed
fertilizers, organic fertilizers, or a combination of the two.
Comparing processed and organic fertilizers.
Processed fertilizers are manufactured or refined from
natural ingredients to make them more concentrated
and more available to plants. Typically they are pro-
cessed into soluble, ionic forms that will be immedi-
ately available to plants.
Organic fertilizers are natural materials that have under-
gone little or no processing. They include both biologi-
cal (plant and animal) and mineral materials (Table 5).
Organic fertilizers release nutrients through natural
processes in the soil, including chemical weathering
of mineral materials, and biological decomposition of
organic matter. The released nutrients are available to
plants in a water-soluble form. These soluble forms
of nutrients are the same as those supplied by pro-
cessed fertilizers.
Compared with processed fertilizers, organic fertiliz-
ers usually contain smaller amounts of nutrients, and
they release nutrients more slowly. You need to apply
larger amounts of organic fertilizers, but their effects
last longer. Organic fertilizers contain a variety of
nutrients, but the amounts are not always balanced
according to plant needs.
Using organic fertilizers recycles materials that other-
wise would be discarded as waste. Production of pro-
cessed fertilizers, on the other hand, can create waste
and use substantial amounts of energy.
Slow release of nutrients. Organic fertilizers are
slow-release fertilizers because their nutrients become
available to plants during the course of the growing
season through the nutrient cycling process described
above. The rate of release of nutrients from organic
materials depends on the activity of soil microorgan-
isms, just as it does for soil organic matter. Tempera-
Soil Management for Small Farms — 11
Table 5. Comparing organic and processed fertilizers.
Organic fertilizers Processed fertilizers
Source Natural materials; little or no processing Manufactured or extracted from
natural materials, often undergoing
extensive processing
Examples Manure, cottonseed meal, rock Ammonium sulfate, processed urea,
phosphate, fish by-products, ground potassium chloride
limestone
Nutrient Usually slow-release; nutrients are Nutrients usually are immediately
Availability released by biological and chemical available to plants
processes in soil
Nutrient Usually low concentration Usually high concentration
Concentration
ture and moisture conditions that favor plant growth also
favor the release of nutrients from organic fertilizers.
Some organic fertilizers contain immediately avail-
able nutrients as well as slow-release nutrients. These
materials can supply nutrients to plants both early in
the season and later. Fresh manure and fish emulsions
are examples of organic fertilizers containing avail-
able nutrients as well as slow-release ones. As manure
ages, the most readily available fraction is lost into
the air or leached into the soil, leaving slow-release
material in the aged manure.
Some material in organic fertilizers decays so slowly
that the nutrients do not become available the first
season after application. Repeated application of organic
fertilizers builds up a pool of material that releases
nutrients very slowly. In the long run, this will decrease
the amount of fertilizer needed each year.
Fertilizer Labels
The labels on fertilizer containers tell the amount of
each of the three primary nutrients in the fertilizer,
expressed as a percent of total fertilizer weight: Nitrogen
(N) is always listed first, phosphorus (P) second, and
potassium (K) third. Historically, fertilizer labels have
not listed the amount of phosphorus as P, but as units
of P
2
O
5
.
1
This convention is still used today for
fertilizer labels and recommendations, even though
there is no practical reason for doing so, except that
people are accustomed to it. Similarly, fertilizer labels
list potassium as K
2
O. For example, a bag of fertilizer
labeled 5-10-10 contains 5% nitrogen expressed as
N, 10% phosphorus expressed as P
2
O
5
, and 10% potas-
sium expressed as K
2
O. This information is the called
the fertilizer analysis.
For processed fertilizers the analysis guarantees the
amount of available nutrients in the fertilizer. For
organic fertilizers the analysis is for the total amount
of nutrients rather than available nutrients. The amount
of available nutrients will be less than the total, because
nutrients in most organic fertilizers are initially unavail-
able to plants and are released slowly.
Examples of Processed Fertilizers
Nitrogen. The raw material for processed nitrogen
fertilizer is nitrogen gas from the atmosphere. The
manufacturing process is the chemical equivalent
of biological nitrogen fixation, and it requires a sub-
stantial amount of fossil fuel energy. Examples of
processed nitrogen fertilizers are listed in Table 6.
Phosphorus and potassium. Processed phosphorus
fertilizers (Table 7) come from phosphate rock. The
rock is treated with acid, releasing the phosphorus
into plant-available forms.
12 — Soil Management for Small Farms
1
If you need to convert from P to P
2
O
5
, the conversion is 1lb P = 2.3 lb
P
2
O
5
. For potassium the conversion is 1lb K = 1.2 lb K
2
O.
Soil Management for Small Farms — 13
The most common raw material for potassium fertiliz-
ers is sylvinite (Table 7), a mixture of sodium chloride
and potassium chloride salts. The potassium in sylvinite
is already in soluble form, but the sylvinite is treated
to remove the sodium salts, making it suitable to use
as a fertilizer. Some other potassium fertilizers are
potassium sulfate salts, which supply sulfur as well
as potassium.
Mixed fertilizers. Mixed fertilizers contain all three
primary nutrients blended in varying ratios. Many
farmers find these are more convenient to use than
fertilizers providing individual nutrients, although
they tend to be more expensive. Use soil test results
and recommendations from Cooperative Extension
publications to determine which ratios best meet
your needs.
Common Organic Fertilizers
Animal manure. Manure is a good source of plant
nutrients and organic matter, and it is readily avail-
able for many growers. Properly managed manure
applications recycle nutrients to crops, improve soil
quality, and protect water quality. Animal manures
vary widely in nutrient content and nutrient availabil-
ity, depending on the type of animal that produced
Table 6. Examples of processed nitrogen fertilizer materials.
Material Analysis Comments
Urea 46-0-0 Rapidly converted to ammonium in soil.
Ammonium sulfate 21-0-0 Also contains 24% available sulfur. Used
with acid-loving plants such as blueberries.
Diammonium phosphate 18-46-0 Used in mixed N-P-K fertilizers as a source of
nitrogen and phosphorus.
Ammonium nitrate 34-0-0 Contains N in nitrate and ammonium forms.
Table 7. Examples of processed phosphorus and potassium fertilizers.
Material Typical Analysis Comments
Triple superphosphate 0-46-0 Concentrated phosphorus fertilizer.
Monoammonium phosphate 11-52-0 Used in mixed fertilizers as a source of
nitrogen and phosphorus. Also used as a
starter fertilizer.
Diammonium phosphate 18-46-0 Used in mixed fertilizers as a source of
nitrogen and phosphorus.
Potassium chloride 0-0-60 Concentrated source of potassium.
Potassium magnesium sulfate 0-0-22 Also contains 11% magnesium and 18%
sulfur.
Potassium sulfate 0-0-50 Also contains 18% sulfur.

the manure and the age and handling of the manure.
Farmers must be able to understand and reduce that
variability to make best agronomic and environmental
use of manure.
This section is a brief introduction to using manure
as a fertilizer. For details on manure use, including
manure testing, determining application rates, and
spreader calibration, see Fertilizing with Manure,
PNW0533.
• Sources of manure. You can obtain manure in bulk
from manure processors, organic fertilizer dealers,
or directly from livestock producers. Manure from
processors is often more uniform and will have a
guaranteed nutrient analysis. Manure processors
often compost manure to destroy pathogens and
weed seeds. Manure from livestock producers is
usually less expensive, or even free, but it may
be more variable in quality and may not have an
analysis. If the manure is not composted or well
aged, it may contain weed seeds and pathogens.
Be sure to read the sidebar on manure safety
before using fresh manures.
• Nutrient content. Not knowing the nutrient content
of manure can lead to large errors in application
rate. We strongly advise that you test the manure
you plan to use. If you buy manure from a commer-
cial source they should be able to provide you with
nutrient test values, and you would not need to do
further testing.
In the absence of test values, use the published
values in Table 8 as a starting point. Remember
that these are average values and they may not
accurately represent your situation.
• Applying manure. Manure application rates are
usually based on N because N is usually the nutrient
needed in the largest quantity for crop growth.
Manure is not like commercial fertilizer in that it
does not come with a guaranteed N availability.
Nitrogen availability from manure varies greatly,
depending on the type of animal, type and amount
of bedding, and age and storage of manure. There
is no simple test to determine N availability for an
individual manure sample. Use Table 8 as a guide-
line for estimating N availability.
Horse manure or other manures with lots of woody
bedding may remove available nitrogen from the
soil (N immobilization), rather than supply nitro-
gen for crop growth. Woody material contains so
little N that microorganisms must use soil N to
supply their metabolic needs as they break the
material down. Expect nitrogen immobilization
from manures containing less than 1% N.
Experiment with manure applications and observe
the performance of your crops to fine-tune your
application rate. It’s better to be conservative with
your application rate and add more nutrients if the
crops appear deficient.
14 — Soil Management for Small Farms
Table 8. Typical nutrient content, solids content, bulk density, and estimate of nitrogen
availability for animal manure at the time of application.
Bulk Nitrogen
Type N P
2
O
5
K
2
O Solids density availability
lb / ton as-is % lb/cu yard %
Broiler with litter 73 63 46 70 900 40–70
Laying hen 37 56 32 40 1400 40–70
Sheep 18 9 24 28 1400 25–50
Beef 12 6 12 23 1400 20–40
Dry stack dairy 9 4 13 35 1400 20–40
Separated dairy solids 5 2 2 19 1100 0–20
Horse 9 6 11 37 1400 0–20
Soil Management for Small Farms — 15
The best of manure application estimates will not
be useful if you don’t know how much you’re
applying once you get into the field. You will need
a spreader with capacity matched to the size of
your farm, and you will need to calibrate it so that
you have confidence in your application rates. See
Fertilizing with Manure, PNW0533, for details on
estimating nutrient availability, application rates,
and spreader calibration.
• Timing manure applications. The best time to
apply manure to row crops is in the spring before
Fresh manure sometimes contains disease-causing
pathogens that can contaminate produce. Salmo-
nella bacteria are among the most serious pathogens
found in animal manure. Pathogenic strains of E. coli
bacteria can be present in cattle manure. Manure
from swine and carnivores can contain helminths,
which are parasitic worms.
These pathogens are not taken up into plant tissue,
but they can adhere to soil on plant roots, or on the
leaves or fruit of low-growing crops. Cooking
destroys pathogens, but raw food carries a risk of
pathogen exposure. Although washing and peeling
raw produce removes most pathogens, some may
remain. The risk from pathogens is greatest for root
crops (e.g., carrots and radishes) or leaf crops (e.g.,
lettuce or spinach), where the edible part touches
planting. You also can apply manure in the fall, but
some of the nutrients will be lost during the winter
if you apply manure to bare ground. Environmental
risks of leaching and runoff also increase. If you
do apply manure in the fall, apply it early, and plant
a cover crop to help capture nutrients and prevent
runoff. You can apply manure to pastures from late
February through mid October in most parts of
western Washington, as long as the applications
are at moderate rates.
Biosolids. Biosolids are a by-product of wastewater
treatment. They are processed wastewater solids that
meet federal and state criteria for application to land.
A common form of biosolids is a spongy, black sub-
stance called “cake.” Biosolids cake is about 20 to 25%
dry matter and 75 to 80% water. It typically contains
about 3 to 6% nitrogen and 2 to 3% phosphorus on
a dry weight basis, plus small amounts of potassium
and trace elements. Some of the nitrogen in biosolids
is immediately available to plants. The rest is released
slowly. Most of the biosolids produced in Washington
are used to fertilize agricultural and forest crops. Typical
agricultural application rates range from 2 to 5 dry
tons per acre depending on the nitrogen content of the
biosolids and the nitrogen requirement of the crop.
the soil. The risk is negligible for crops such as sweet
corn, which does not come in contact with the soil, or
for any crop that is cooked thoroughly.
Avoid using fresh manure where you grow high-risk
crops. Composting manure at high temperatures will
kill pathogens, but you need careful quality control to
make sure that all of the manure reaches conditions
for pathogen kill. Refer to the On-Farm Composting
Handbook for details on composting procedures.
Commercial manure composts are composted under
conditions to destroy pathogens. Bacterial pathogens
die naturally in the environment during a period of
weeks or months, and well-aged manure should not
contain them. Helminths in swine manure can persist
in soil for years, however. High temperature com-
posting will kill helminths.
Using Manure Safely

Some commercial and municipal composters use bio-
solids as an ingredient in making compost. Biosolids
composts behave like other composts, slowly releas-
ing nutrients. They are a good source of organic matter
and will provide small amounts of nutrients to plants.
There are two classes of biosolids based on pathogen
removal. Class A biosolids include biosolids composts
and heat-treated biosolids. They are virtually free of
pathogens, and are safe to use on any crop.
Class B biosolids are processed to reduce, but not
eliminate pathogens. Pathogens remaining in Class B
biosolids are similar to those in fresh manure. After
land application any remaining pathogens in class B
biosolids are killed by exposure to sunlight, drying,
soil microorganisms and other environmental factors.
To allow time for the pathogens to die off, federal
regulations require waiting periods between the
application of class B biosolids and the harvest of
crops. Waiting periods are longest for crops where
the edible part touches the soil (more than 1 year for
aboveground crops and more than 3 years for root
crops). Because of the length of the waiting periods,
it is usually impractical to use Class B biosolids on
vegetable crops. The most commonly used crops for
Class B biosolids application are grain crops and
pastures, which have much shorter waiting periods.
Biosolids contain small amounts of trace elements.
Some trace elements are micronutrients (such as zinc,
copper, and molybdenum) which can benefit crops.
Other trace elements (such as lead and cadmium) have
no known beneficial effects. Large amounts of trace
elements can be toxic to crops, animals, or humans.
Levels of trace elements in biosolids produced in
Washington are low compared with federal standards.
When growers apply Washington biosolids at rates to
meet crop nitrogen requirements, the trace element
accumulations in the crop are insignificant.
You can usually obtain biosolids free or at low cost
from wastewater treatment plants. In many cases the
treatment plant or their contractor will apply the bio-
solids for you. Even though the risks associated with
biosolids use are no greater than risks with manure,
the Department of Ecology requires that wastewater
treatment operators obtain permits for biosolids applica-
tion. As a farmer you do not have to apply for a permit,
but the permit may affect your farm management. For
16 — Soil Management for Small Farms
example, the permit will specify application rates and
timing, and application buffers to ditches, streams,
and property boundaries.
If you consider using biosolids, ask the following
questions:
• Are the biosolids Class A or Class B?
• What is the content of nutrients and trace ele-
ments in the biosolids?
• Are they a slurry (more odor) or a solid?
• What site management and waiting period
requirements are there?
• When will the biosolids be available, and does
the timing fit in with your farm management?
• Who will apply the biosolids and what equipment
will they use?
• Will buyers accept crops treated with biosolids?
(Some food processors and consumers will not buy
vegetable crops fertilized with biosolids, but buyer
acceptance is less likely an issue for grain crops.)
Although biosolids behave like organic fertilizers,
they are not certified as such, and certified organic
farmers should not use biosolids. Their best use is
for grain crops and pastures on farms that do not
seek organic certification. For more information
on biosolids, read the extension bulletin PNW0508,
Fertilizing with Biosolids.
Commercial organic fertilizers. Many organic by-
products and some unprocessed minerals are sold as
commercial organic fertilizers. Table 9 shows approxi-
mate nutrient contents of some of these materials.
Numbers represent total nutrient contents; because
most are slow-release fertilizers, not all of the nutri-
ents will be available the same year they are applied.
The table shows that each fertilizer contains one main
nutrient. The other nutrients are present in smaller
amounts. Several companies produce balanced organic
fertilizers, a combination of materials blended into a
single product that provides all of the primary nutri-
ents.
Choosing organic fertilizers. Choosing organic
fertilizers involves tradeoffs in cost and convenience.
Farmyard manure is usually inexpensive or free, but it
is less convenient than packaged, commercial materi-
als. If you or your neighbors have livestock, it makes
both environmental and economic sense to recycle
the manure produced by the livestock.
Soil Management for Small Farms — 17
Commercial organic fertilizers can be expensive, but
you may choose them where convenience or quick
availability of nutrients is important or for small areas
of land under intensive production. The cost per pound
of nutrients in organic fertilizers varies widely, depend-
ing on the type of material, the concentration of nutri-
ents, and the size of the package. Compare costs and
nutrient availability when shopping for organic
fertilizers.
Estimating How Much Fertilizer to Use
The goal of applying fertilizer is to supply enough
nutrients to meet plant needs, without accumulation
of excess nutrients that could harm water quality.
Farmers should have a regular soil testing program
to assess nutrient status and to plan fertilizer
applications.
Soil tests. A soil test will give you (1) information on
the levels of nutrients in your soil, and (2) a recommen-
dation for how much fertilizer to add each year based
on your soil test results and the crops you are grow-
ing. You don’t need to test each field every year. You
can rotate your tests around the farm, testing each
field at least once every 2 to 3 years.
A basic soil test typically includes the following
nutrients: phosphorus, potassium, calcium, magne-
sium, and boron. The test also includes soil pH and
a recommendation for lime if needed. Many soil test
labs don’t test routinely for nitrogen because there is
no simple, reliable test for predicting nitrogen avail-
ability in soils. The lab will give a nitrogen recom-
mendation, however, based on the crops you are
growing and information you provide about the soil
(such as whether there is a history of manure applica-
tions that would increase soil available nitrogen).
Some specialized nitrogen tests are done, such as the
pre-sidedress nitrate test for corn (see Oregon State
University bulletin EM 8650), but samples for these
tests are collected at different times from the basic test.
The best way to take a soil sample is to collect multiple
cores (at least 15) from a field, air-dry them, and mix
the cores together well. Use a cylindrical soil-sam-
pling probe to get uniform samples. Send about a pint
of the dried, mixed sample to the lab. The samples
you collect should be from the top foot (0 to 12-inch
depth) of your soil. Avoid atypical areas such as the
site of an old manure pile, burn pile, or building, or
areas that are unusually wet or eroded.
Farmers generally collect different samples for each
field, crop, and soil type. If you are growing a large
variety of crops on a small acreage, it will not be
economical to do a soil test for each crop, and you
will want to group crops for soil tests. For more infor-
mation on sample collection, see Oregon State Univer-
sity Extension Bulletin EC 628, How To Take a Soil
Sample…And Why, available on the OSU publications
web site at http://eesc.orst.edu, or University of Idaho
Bulletin 704, Soil Sampling, available on the UIdaho
web site at http://info.ag.uidaho.edu/. For information
on interpreting soil tests see OSU bulletin EC 1478,
Soil Test Interpretation Guide, available on the OSU
web site listed above.
Washington State University and Oregon State Univer-
sity no longer test soils, but private labs in both states
do. Cooperative Extension county offices have lists
of testing labs. If you have not worked with a lab
before, call them to make sure they are set up to test
and make recommendations for agricultural soils.
Table 9. Total nitrogen, phosphate and
potash content typical of some organic
fertilizers.
Material % Nitrogen % P
2
O
5
% K
2
O
Cottonseed Meal 6–7 2 1
Blood Meal
1
12–15 1 1
Alfalfa 2 0.5 2
Bat Guano
1
10 3 1
Fish Meal
1
10 4 0
Fish Emulsions
1
3–5 1 1
Bone Meal 1–4 12–24 0
Rock Phosphate
2
0 25–30 0
(only 2–3% available)
Greensand 0 0 3–7
Kelp Meal 1 0.1 2–5
1
These materials contain a substantial amount of quickly
available nitrogen that plants can use early in the season.
2
Very low availability. Useful only in acid soils.

Ask the lab:
• Do you routinely test soils for plant nutrients and
pH?
• Do you use WSU or OSU test methods and
fertilizer guides?
• Do you give recommendations for fertilizer
applications?
• Are there forms to complete? What information
do you need?
• How should the sample be packaged and sent?
• How much does a test cost?
• How quickly will you send results?
Extension publications. Extension publications are a
good source of information on crop nutrient needs.
Use them together with soil test results for planning
fertilizer applications. Check other bulletins in the
Farming West of the Cascades series, or use the WSU
publications web site (caheinfo.wsu.edu) or the OSU
site (eesc.orst.edu) to find appropriate bulletins.
Fertilizer calculations. Fertilizer recommendations
are usually given in pounds of nutrient (such as nitro-
gen) per acre. You will need to convert the fertilizer
recommendations from pounds of nutrient to actual
pounds of fertilizer.
Example
You plan to make a mid-season application of 100 lb/
acre of nitrogen to your corn crop. You are growing 5
acres of corn, using urea (46-0-0).
1. Divide the amount of nitrogen recommended for
1 acre (100 lb) by the fraction of nitrogen in the
fertilizer (46% or .46).
100 lb N/acre / .46 = 218 lb urea/acre
2. Calculate the total amount of fertilizer needed by
multiplying the area of your field by the fertilizer
rate calculated in step 1:
218 lb/acre x 5 acres = 1,090 lb urea
If you are growing crops intensively on a small area
of land, the acre-based calculation for each crop may
not be convenient. To convert the calculations to units
per 1,000 square feet, divide the recommendations per
acre by 44.
Example for a Small Area
You are growing carrots and plan to make a nitrogen
application of 80 lb N/acre. Your carrot bed covers
2,000 square feet and you are using urea (46-0-0).
1. Divide the amount of nitrogen recommended for
1 acre (80 lb) by the fraction of nitrogen in the
fertilizer (46% or .46).
80 lb N/acre / .46 = 174 lb urea/acre
2. Divide the urea rate by 44 to calculate the amount
of urea needed per 1,000 square feet.
174 lb urea/acre / 44 = 4.0 lb urea/1,000 square feet
3. Calculate the total amount of fertilizer needed by
multiplying the area of your field (in 1,000 square
foot units) by the fertilizer rate calculated in step 2.
(4.0 lb urea/1,000 square feet) x 2 = 8.0 lb urea
Tips for Estimating Organic Fertilizer Rates
Estimating how much organic fertilizer to use can be
a challenge because you must estimate the availability
of the nutrients in the organic fertilizer.
• Organic fertilizers having large proportions of
available nutrients (such as bat guano and fish
emulsions) can be substituted in direct proportion
for processed fertilizers.
• For other commercial organic fertilizers, apply
according to their nutrient availability. Composts,
rock phosphate, and plant residues generally have
lower nutrient availability than more concentrated
animal products (bloodmeal, bone meal, and
chicken manure). If you use packaged organic
fertilizers, the recommendations on the package
often are a good guideline for application rates.
Check the recommendations against other prod-
ucts to make sure they seem reasonable.
• The nutrient concentration and availability in
farmyard manures varies widely depending on
the type of manure and its handling. For guide-
lines for determining appropriate application
rates for different types of manures see Fertilizing
with Manure, PNW0533.
18 — Soil Management for Small Farms

• Whatever fertilizer you use, observe your crops
carefully. It is sometimes hard to estimate how
much organic fertilizer to use. Lush plant growth
and delayed fruiting and flowering are signs of
high amounts of available nitrogen, indicating
overfertilization. You can experiment with different
fertilizer rates in different rows and see if you
notice differences. Plan your experiment care-
fully, so you are confident that any results come
from the fertilizer rates, rather than differences
in soil, watering, sunlight, or other management.
• Use soil testing to track changes in nutrient
availability and modify application rates.
Timing Fertilizer Applications
In most cases, the best time to apply fertilizers is close
to the time the plant needs the nutrients. Proper timing
of applications reduces the potential for loss of nutrients
before they are taken up by the plants. Loss of nutri-
ents is not only inefficient, but the lost nutrients may
become contaminants in groundwater or surface water.
Plants need the largest amount of nutrients when they
are growing most rapidly—early to midsummer for
corn and squash, earlier for spring plantings of lettuce
and other greens. Plants also need available nutrients
(especially phosphorus) shortly after seeding and
transplanting. For a long-season crop such as corn,
farmers often add a small amount of fertilizer as a
starter at the time of seeding and a larger amount in
early summer, just before the period of rapid growth.
If the entire application was made in the spring, some
of the nutrients (especially nitrogen) could be lost by
leaching before the plant was ready to use them.
When using organic fertilizers, a single application
is usually adequate because nutrient release occurs
throughout the growing season. If you apply organic
fertilizers to a crop that matures early, the crop will
not take up nutrients that are released from the fertilizer
during the late summer and fall. Nitrogen released
after crop maturity is likely to leach into groundwater
during the winter. Planting cover crops between the
rows or immediately after crop harvest can capture
some of the nutrients released late in the season by
organic fertilizers.
For perennial plants, timing depends on the growth
habit of the plant. Blueberries, for example, benefit
most from fertilizer applied early in the season at
budbreak, while June-bearing strawberries are fertil-
ized after harvest. For crop-specific information on
timing fertilizer applications, refer to the appropriate
extension bulletins.
Calibrating Fertilizer Spreaders
Depending on the size and type of your operation,
the type of spreader you use will vary. If you have
a small, intensively managed crop, you may apply
fertilizer by hand. For a larger area, a hand-operated
whirlybird applicator may work, or you may use a
broadcast or band applicator towed behind a tractor.
Whatever equipment you use, be sure to calibrate it
to apply the appropriate amount of fertilizer.
For tractor-operated applicators you control the rate
by adjusting the fertilizer settings.
1. Select a setting that is likely to be close to your
desired rate, and place a known weight of fertil-
izer in the spreader.
2. Measure a known distance (50 or 100 feet is
adequate) and drive the spreader over that distance.
3. At the end of the run, weigh the fertilizer remain-
ing in the spreader. The difference between the
initial and final weights is the amount spread.
4. Calculate the area spread by multiplying the
distance traveled by the width spread. Convert
into acres by dividing by 43,560.
Soil Management for Small Farms — 19

5. Divide the weight of fertilizer applied by the
application area in acres. This is the application
rate in lb/acre.
6. Compare with your target rate.
7. Adjust the settings as needed and repeat the
process until you are within 10% of the desired
fertilizer rate.
8. Record the settings for future use.
Example
1. You need to apply urea at a rate of 218 lb/acre.
Your spreader width is 5 feet, and the length of
your test run is 100 feet. You apply to the test
area and use 2.1 lb of urea.
2. The area spread is 5 ft x 100 ft = 500 square feet
3. The area spread (in acres) is 500 / 43560 = 0.0115
acre
4. The application rate in lb/acre is 2.1 / 0.0115 =
183 lb/acre
5. The difference from the target rate is 218 - 183 =
35 lb /acre
6. This is more than 10% below the target so you
will need to open the settings and test again.
You can adapt the above method to work with hand-
operated whirlybird spreaders. It may be more conve-
nient to calculate on the basis of 1,000 square foot
units rather than acre units. You can adjust rates with
whirlybird spreaders by changing the settings or chang-
ing your walking speed.
You can also calibrate a spreader by placing it on
blocks so that the wheels can spin freely. Place fertilizer
in the spreader, and place a tarp beneath the spreader.
Turn the wheels, counting the number of turns. Deter-
mine the distance traveled by multiplying the circum-
ference of the wheel by the number of turns. Weigh
the fertilizer on the tarp, and continue with the above
calculation. For calibrating manure spreaders see
Fertilizing with Manure, PNW0533.
If you change fertilizer formulation or application
rate, you will need to recalibrate the spreader for the
new conditions.
Adding Organic Matter
Organic matter builds and stabilizes soil structure,
improving the porosity, infiltration, and drainage of
the soil, and reducing erosion. It holds water and
nutrients for plants and soil organisms. Organic matter
also is a long-term, slow-release storehouse of nitro-
gen, phosphorus, and sulfur.
The value of organic amendments varies, depending
on their nitrogen content, or more specifically on their
carbon to nitrogen (C:N) ratio. Organic materials that
have a low C:N ratio, such as undiluted manure or
bloodmeal, are rich in nitrogen. They are a good source
of nutrients, but growers must use them sparingly to
avoid overfertilization.
Materials with an intermediate C:N ratio (including
many composts, leaf mulches, and cover crop residues)
have lower nutrient availability. They are the best
materials to replenish soil organic matter. Because
they are relatively low in available nutrients, they
can be added to the soil in large amounts.
Materials with a high C:N ratio (such as straw, bark,
and sawdust) contain so little nitrogen that they will
reduce levels of available nitrogen when they are
mixed into the soil. Soil microorganisms use available
nitrogen from the soil when they decompose these
materials, leaving little nitrogen for the plants. This
process is called immobilization and it results in
nitrogen deficiency. If you amend your soil with
materials with a high C:N ratio, you will need to add
extra nitrogen fertilizer to compensate for immobili-
zation. The best use for these materials is as mulches
around perennial crops or in walkways. They will not
immobilize nitrogen until you mix them into the soil.
Green Manure
Green manures are cover crops grown specifically to
be tilled into the soil. Planting green manure is a way
to grow your own organic matter. The value of cover
crops goes beyond their contribution of organic
matter. Cover crops also can do the following:
20 — Soil Management for Small Farms
• Capture and recycle nutrients that otherwise
would be lost by leaching during the winter.
• Protect the soil surface from rainfall impact
during the winter.
• Reduce runoff and erosion.
• Help suppress weeds.
• Supply nitrogen (if legumes are grown).
No one cover crop provides all of these benefits
(Table 10). Deciding which cover crop or crop
combination to grow depends on which benefits
are most important to you, and which cover crops
fit best into your farm plan.
Farmers usually plant cover crops in the fall and till
them as green manure in the spring, before planting.
The earlier cover crops are planted, the more benefits
they will provide. Research in western Washington
showed that cereal rye planted in September captured
three times the amount of nitrogen as an October
planting. Legumes such as vetch and crimson clover
Table 10. Examples of cover crops grown
in Washington.
Cereal Rye
Very hardy, grows quickly, matures rapidly in
spring. Helps suppress winter weeds, protects
soil surface from raindrop impact and erosion.
Winter Wheat
Leafy, covers soil well, matures slowly. Helps
suppress winter weeds, protects soil surface
from raindrop impact and erosion.
Hairy Vetch
Legume, fixes nitrogen, starts slowly, grows
quickly in spring, good companion crop for
cereal rye.
Crimson Clover
Legume, fixes nitrogen, slower growth than
vetch.
Buckwheat
Fast growing, frost-sensitive, ready to till in 30
days. Helps suppress weeds. Produces biomass
quickly. Use as a summer cover crop.
need an early start to cover the soil before cold weather
arrives.
You will not get much benefit from cover crops planted
after October. Plant cover crops in areas you harvest
early, and consider applying compost mulches or using
minimum tillage in areas you harvest later. You can
also start cover crops between rows of late crops
where space allows.
Till cover crops into the soil before they flower. After
flowering, the plants become woody and decline in
quality. Also, tilling the crop into the soil becomes
more difficult if the plants grow too large.
The organic matter benefits from cover crops last only
about one year. Where possible, make cover crops an
annual part of your crop rotation. The WSU Cooperative
Extension Bulletin EB1824, Cover Crops for Gardens
in Western Washington and Oregon, gives details on
choosing and managing cover crops in areas west of
the Cascades. It is useful for small-acreage farmers as
well as gardeners. For more details on specific cover
crops, refer to the Oregon State University Extension
Service cover crops series. These publications are
available on the Web (see page 18 for WSU and OSU
publications web addresses).
Composts
Composts provide an excellent source of organic
matter. They also supply a modest amount of nutrients
released slowly over the long term. You need to apply
Vetch
Soil Management for Small Farms — 21

large amounts of compost (100 to 200 yards per acre)
to see substantial benefits, so the cost of purchased
composts can be prohibitive for all but small areas
of intensively managed crops.
Commercial composts. Yard debris is the major
raw material in most commercial composts sold in
Washington and Oregon. Commercial composts may
also contain animal manure, biosolids, food waste,
or wood waste. Commercial composts are made on a
large scale, with aeration and/or frequent turning to
meet time and temperature requirements to kill weed
seeds, plant pathogens, and human pathogens. They
are high quality materials, but they are usually too
expensive for general agricultural use.
On-farm composting. An alternative to commercial
composts is making compost on your farm using crop
residues, manure, or other appropriate materials gen-
erated on the farm. In some counties you can import
material to compost and use on-farm without a permit,
while other counties require a permit. If you sell or
distribute your compost off-farm you will need a permit.
Contact your local health department if you have
questions about composting permits.
To produce high-quality compost you need to gener-
ate high temperatures within the pile and turn the pile
frequently to make sure all material is exposed to high
temperatures. High-temperature composting demands
time and attention, and it’s not for every farmer. Com-
posting will still occur at lower temperatures, but you
will not get a complete kill of weed seeds or patho-
gens. You can still produce suitable compost at lower
temperatures, as long as you avoid raw materials that
are full of weed seeds or pathogens.
For detailed information about on-farm composting,
including raw materials, methods, and equipment,
refer to the On-Farm Composting Handbook, avail-
able from the Northeast Regional Agricultural Engi-
neering Service, Cornell University Cooperative
Extension, Ithaca, New York, 14853-5701.
Partially Composted Yard Debris
Some commercial composters will provide partially
composted yard debris to farmers during periods of
peak flow. These periods usually occur during the
spring when homeowners ship large volumes of yard
debris to composters. If composters can divert some
of the flow to farms, they can avoid overloading their
composting facilities. The timing is usually good for
farmers because it occurs when they are preparing
land for spring crops.
Research in western Washington has shown that
partially composted yard debris is a good source of
nutrients and organic matter. Application rates of 20
to 30 dry tons per acre per year can supply all the
nutrients needed for a corn crop and increase soil
organic matter levels. Because the material is not fully
composted, there is some risk of weeds, but no weed
problems have been observed in the experiments and
on-farm demonstrations done by researchers at WSU
Puyallup.
Yard debris is inexpensive for farmers, but prices
could rise depending on supply and demand. Process-
ing of yard debris differs, depending on the facility.
Some yard debris is composted actively for a few
days before delivery to the farm, while others do not
have active composting. Contact local composters
to find out if they have a yard debris program for
agriculture. Find out about their procedures, costs,
and timing to see if it fits into your farm plan.
Soil pH and Liming
Soil pH measures the acidity or alkalinity of a soil.
A pH of 7 is neutral—where acidity and alkalinity
are balanced. Acidity increases by a factor of 10 with
each 1 unit drop in pH below 7. For example, a pH
of 5.5 is ten times as acidic as a pH of 6.5. Alkalinity
increases by a factor of 10 with each 1-unit change in
pH above 7. Native soil pH depends on the minerals
present in the soil and the amount of rainfall. Soils
tend to be near neutral in the low rainfall areas of
western Washington (around Sequim, Port Townsend,
and Coupeville) and acid in the moderate to high
rainfall areas. Farming practices also affect soil pH;
many nitrogen fertilizers tend to reduce soil pH,
while liming increases soil pH.
Soil pH influences plant growth in three ways:
• by affecting availability of plant nutrients
• by affecting availability of toxic metals
• by affecting the activity of soil microorganisms,
which in turn affects nutrient cycling and disease
risk.
22 — Soil Management for Small Farms

The availability of phosphorus decreases in acid soils,
while the availability of iron increases. In alkaline
soils, the availability of iron and zinc can be quite
low. In acid soils aluminum availability increases.
Aluminum is one of the most common elements in
soil, but it is not a plant nutrient, and it is toxic to
plants in high concentrations. Very little aluminum
is in solution in soils above pH 6 and it causes no
problems to plants. As pH declines and aluminum
availability increases, aluminum toxicity becomes a
problem.
Soil pH affects microbes in a similar way. The most
numerous and diverse microbial populations exist in
the middle of the pH range; fewer organisms are adapted
to strongly acid or strongly alkaline soils. Nutrient
cycling is slower in acid and alkaline soils because
of reduced microbial populations. Soil pH also affects
pathogenic microbes, and growers can adjust pH to
manage some plant diseases.
Many crops perform best between pH 6 and 7.5, but
some (such as blueberries) are adapted to more strongly
acid soils. Before amending the soil to adjust pH, you
must know the preferred pH ranges of your crops.
Increasing soil pH with lime. Lime is ground lime-
stone, a rock containing calcium carbonate. It is a
certified organic amendment, suitable for use in organic
agriculture. Lime raises the pH of acid soils and sup-
plies the essential nutrient, calcium. Dolomitic lime
contains magnesium as well as calcium, and it can
correct magnesium deficiencies in soil as well as raise
soil pH. Dolomitic lime is more expensive and slower
acting than agricultural lime, so use it only when you
need to.
A basic soil test gives you a lime recommendation in
tons of agricultural lime (calcium carbonate) per acre.
Lime is a slow-release material. A fall application will
benefit a spring crop. Do not lime areas where you
grow acid-loving plants.
Gypsum (calcium sulfate) is not a substitute for lime.
It provides calcium and sulfur to soils, but it has little
effect on soil pH. Gypsum has been promoted as a
soil amendment to improve soil structure, but it does
not work in our environment. Gypsum improves
structure only when the problem results from excess
sodium in the soil, a rare condition west of the
Cascades. Use organic amendments to improve soil
structure, as described earlier.
Decreasing soil pH. Elemental sulfur lowers the pH
of a soil. If your soil pH is too high for acid-loving
crops that you are growing, ask your soil test lab for
an acidification recommendation. Ammonium sulfate
fertilizer also lowers pH, but the effect is not as fast
as for sulfur. Urea reduces pH slowly, as do some
organic fertilizers.
About the Author
Craig Cogger is an Extension Soil Scientist, Depart-
ment of Crop and Soil Sciences, Washington State
University, Puyallup, WA. He specializes in research
and extension programs in the use of animal manures,
composts, and other organic byproducts for crop
production.
Soil Management for Small Farms — 23

The series Farming West of the Cascades is a project of the WSU Food and Farm Connections Team. The Food and Farm
Connections Team is a group of Cooperative Extension faculty and staff seeking to promote and enhance sustainable,
community-based food and fiber systems through research, education, and partnerships. The team is supported by the WSU
Center for Sustaining Agriculture and Natural Resources (CSANR). For more information about the team or CSANR,
visit our website at <http://foodfarm.wsu.edu>, or call (253) 445-4514.
Funding for this project was provided by WSU Cooperative Extension and the King County Agriculture Commission.
Copyright 2000, Washington State University.
A list of WSU publications is available online <http://caheinfo.wsu.edu> or order through the Bulletin office 1-800-723-1763.
Issued by Washington State University Cooperative Extension, Oregon State University Extension Service, University of
Idaho Cooperative Extension System, and the U. S. Department of Agriculture in furtherance of the Acts of May 8 and June
30, 1914. Cooperative Extension programs and policies comply with federal and state laws and regulations on nondis-
crimination regarding race, sex, religion, age, color, creed, national or ethnic origin; physical, mental, or sensory disability;
marital status, sexual orientation, and status as a Vietnam-era or disabled veteran. Evidence of noncompliance may be
reported through your local Cooperative Extension office. Trade names have been used to simplify information; no
endorsement is intended. Published July 2000. Subject code 371. C. EB1895
K ING C OUNTY
Department of Natural Resources
King County Agriculture Commission
COOPERATIVE EXTENSION
24 — Soil Management for Small Farms
