
Global Guidelines for Acute Flaccid Paralysis (AFP) and Poliovirus Surveillance
1
GLOBAL GUIDELINES
for acute flaccid paralysis (AFP) surveillance
in the context of poliovirus eradication
Pre-
publication
version

2
GLOBAL GUIDELINES
for acute flaccid paralysis (AFP) surveillance
in the context of poliovirus eradication

HOLD for WHO copyright info

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
iv
CONTENTS
ACKNOWLEDGEMENTS ....................................................................................................................... v
ACRONYMS AND ABBREVIATIONS .................................................................................................. vi
ABOUT THESE GUIDELINES ............................................................................................................. vii
INTRODUCTION ..................................................................................................................................... 1
1. Poliovirus and poliomyelitis ................................................................................................................. 1
2. Polio eradication .................................................................................................................................. 2
3. Polio surveillance systems .................................................................................................................. 3
PRINCIPLES of AFP surveillance ........................................................................................................ 4
1. Adopting AFP as a reportable syndrome ............................................................................................ 4
2. Testing all stool specimens in a WHO-accredited polio laboratory .................................................... 5
STRATEGIES for AFP surveillance ..................................................................................................... 6
1. Routine (passive) surveillance ............................................................................................................ 6
2. Active surveillance............................................................................................................................... 7
3. Community-based surveillance ......................................................................................................... 13
4. Supplemental strategies for special populations .............................................................................. 15
CASE ACTIVITIES for AFP surveillance ........................................................................................... 18
1. Timely detection ................................................................................................................................ 19
2. Case notification and verification ...................................................................................................... 19
3. Case investigation and validation ..................................................................................................... 20
4. Stool collection and transport to the laboratory ................................................................................ 22
5. AFP contact sampling ....................................................................................................................... 24
6. Laboratory testing and reporting ....................................................................................................... 27
7. 60-day follow-up investigation ........................................................................................................... 30
8. Final AFP case classification ............................................................................................................ 31
MONITORING AFP surveillance ......................................................................................................... 34
1. Data management ............................................................................................................................. 34
2. Monitoring ......................................................................................................................................... 37
3. Evaluation ......................................................................................................................................... 41
SUSTAINING AFP surveillance .......................................................................................................... 42
1. Building a skilled workforce ............................................................................................................... 42
2. Integrating disease surveillance, the future of polio surveillance...................................................... 43
ANNEXES ............................................................................................................................................. 45

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
v
ACKNOWLEDGEMENTS
This document reflects contributions from field, regional and global epidemiologists, laboratorians,
information system specialists and public health and gender experts in a process led by the agency
partners of the Global Polio Eradication Initiative (GPEI): Rotary International, the World Health
Organization (WHO), the U.S. Centers for Disease Control and Prevention (CDC), the United Nations
Children’s Fund (UNICEF), the Bill & Melinda Gates Foundation and Gavi, the Vaccine Alliance.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
vi
ACRONYMS AND ABBREVIATIONS
AEFI Adverse event following immunization
AESI Adverse event of special interest
AFP Acute flaccid paralysis
AFR African Region (WHO)
AMR Region of the Americas (WHO)
AS Active surveillance
AVADAR Auto-Visual AFP Detection and Reporting
aVDPV Ambiguous vaccine-derived poliovirus
bOPV Bivalent oral polio vaccine
CBS Community-based surveillance
CIF Case investigation form
COVID-19 Coronavirus disease (2019)
cVDPV Circulating vaccine-derived poliovirus
cVDPV1 Circulating vaccine-derived poliovirus type 1
cVDPV2 Circulating vaccine-derived poliovirus type 2
cVDPV3 Circulating vaccine-derived poliovirus type 3
DG Director-general (WHO)
EI Essential immunization
EMG Electromyography
EMR Eastern Mediterranean Region (WHO)
EPID Epidemiological identification
ERC Expert Review Committee
ES Environmental surveillance
EUR European Region (WHO)
GACVS Global Advisory Committee on Vaccine
Safety
GBS Guillain-Barré syndrome
GCC Global Commission for the Certification of the
Eradication of Poliomyelitis
GIS Geographic information system
GPEI Global Polio Eradication Initiative
GPLN Global Polio Laboratory Network
GPS Global positioning system
IDP Internally displaced population
IPV Inactivated polio vaccine
ITD Intratypic differentiation
iVDPV Immunodeficiency-associated vaccine-
derived poliovirus
MOH Ministry of Health
mOPV Monovalent oral polio vaccine
mOPV1 Monovalent oral polio vaccine type 1
mOPV2 Monovalent oral polio vaccine type 2
mOPV3 Monovalent oral polio vaccine type 3
NCC National Certification Committee
NEC National Expert Committee
NGO Nongovernmental organization
nOPV Novel oral polio vaccine
nOPV2 Novel oral polio vaccine type 2
NPAFP Non-polio acute flaccid paralysis
NPEC National Polio Expert Committee
NPEV Non-polio enterovirus
OBRA Outbreak response assessment
OPV Oral polio vaccine
PID Primary immunodeficiency disorder
POLIS Polio Information System
RCC Regional Commission for the Certification of
the Eradication of Poliomyelitis
RNA Ribonucleic acid
SEAR South-East Asia Region (WHO)
SIA Supplementary immunization activity
SL Sabin-like
SOPs Standard operating procedures
tOPV Trivalent oral polio vaccine
TORs Terms of reference
VAPP Vaccine-associated paralytic poliomyelitis
VDPV Vaccine-derived poliovirus
VDPV1 Vaccine-derived poliovirus type 1
VDPV2 Vaccine-derived poliovirus type 2
VDPV3 Vaccine-derived poliovirus type 3
VP1 Virus protein 1
VPD Vaccine-preventable disease
VRE Vaccine-related event
WebIFA Web-based information for action
WHO World Health Organization
WPR Western Pacific Region (WHO)
WPV Wild poliovirus
WPV1 Wild poliovirus type 1
WPV2 Wild poliovirus type 2
WPV3 Wild poliovirus type 3

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
vii
ABOUT THESE GUIDELINES
These Global guidelines for acute flaccid paralysis
(AFP) surveillance in the context of poliovirus
eradication are published to replace the 1996 revision
of the Field guide for supplementary activities aimed at
achieving polio eradication.
Since 1996, all regions of the World Health Organization
(WHO) and several polio-endemic countries have produced
their own AFP surveillance guidelines based on the Field guide,
which has served the programme well. These country-level
guidelines can be updated, if deemed necessary, based on
these new guidelines.
The new global guidelines outline well-established strategies
and activities for AFP surveillance to support countries in
attaining and maintaining a surveillance system sensitive
enough to detect the circulation of any polioviruses – wild polioviruses (WPVs), vaccine-derived
polioviruses (VDPVs) and Sabin-like (SL) viruses. The new guidelines also incorporate
recommendations made through a recent series of field guides and job aids that address current
surveillance-related challenges, and present new tools devised to enhance surveillance sensitivity and
increase the speed of detection of polioviruses. In addition, they introduce new indicators that
complement well-established certification standard indicators, such as those aimed at capturing the
timeliness of field activities. Overall, the guidelines stress four cross-cutting issues that remain central to
the success of the polio eradication programme: (1) the speed of poliovirus detection, (2) the quality of
surveillance at the subnational level, (3) the importance of gender equality to polio eradication, and
(4) the need for integrating polio with other vaccine-preventable disease (VPD) programmes.
These guidelines are intended for use by individuals and organizations involved in polio eradication
efforts that include: national polio surveillance and immunization programme managers and staff;
country, regional and global focal points for polio surveillance and immunization at the WHO and the
United Nations Children’s Fund (UNICEF); polio technical advisory bodies; and partners of the Global
Polio Eradication Initiative (GPEI).

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
1
INTRODUCTION
Since its establishment in 1988, the Global Polio Eradication Initiative (GPEI) has made major progress
towards the goal of eradicating wild poliovirus (WPV). Five of six regions as defined by the World Health
Organization (WHO) have been certified as WPV-free: the Region of the Americas, the Western Pacific
Region, the European Region, the South-East Asian Region and the African Region. Of the three WPV
serotypes, the Global Commission for the Certification of the Eradication of Poliomyelitis (GCC) has
certified the global eradication of two serotypes: type 2 and type 3, last reported in 1999 and 2012,
respectively. At the time of this writing (October 2022), only WPV type 1 (WPV1) remains, with two
countries classified as endemic: Afghanistan and Pakistan.
1. Poliovirus and poliomyelitis
Poliomyelitis is a highly contagious disease caused by a
human enterovirus called poliovirus. Poliovirus consists of
a ribonucleic acid (RNA) genome enclosed in a protein
shell, referred to as a capsid. Of the three serotypes of
poliovirus (types 1, 2, and 3), each have a slightly different
capsid protein. Immunity to one serotype does not confer
immunity to the other serotypes.
The virus is most often spread by the faecal-oral route through contact with the faeces of an infected
person, which occurs mostly in areas with poor water, sanitation and hygiene. It can also spread
through droplets from a sneeze or cough (oral-to-oral transmission), though this is less common and
occurs mainly in areas with relatively better hygiene and sanitary conditions. Poliovirus enters through
the mouth and multiplies in the intestine. Infected individuals shed poliovirus into the environment for
several weeks, where it can spread rapidly in the community, especially in areas of poor sanitation.
Poliovirus can interact with its host in two ways:
• Most poliovirus infections are asymptomatic or cause minor illness with mild symptoms without
affecting the central nervous system.
• Less than 1% of poliovirus infections result in paralysis by affecting the central nervous system,
a life-threatening disease called poliomyelitis.
Poliomyelitis cannot be cured but it can be prevented. Vaccination is safe, effective and inexpensive. It
is through the widespread use of the oral poliovirus vaccine (OPV) that the polio eradication effort owes
its success. Unfortunately, in rare circumstances (approximately 1 in 2.7 million doses),
1
the attenuated
Sabin strains in OPV cause vaccine-associated paralytic poliomyelitis (VAPP) in the vaccine recipient or
a close contact.
In addition, in rare occasions through prolonged excretion or transmission, the vaccine virus can
genetically mutate to a form known as vaccine-derived
poliovirus (VDPV), which like WPV reflects the three
serotypes targeted by vaccines. There are three categories
of VDPVs: circulating, immunodeficiency-associated and
ambiguous. VDPVs represent a challenge to polio
eradication and are a focus of the programme in the last
mile to eradication.
2
1
See the fact sheet on types of polioviruses and vaccines, published on the GPEI website (https://polioeradication.org/wp-
content/uploads/2018/07/GPEI-cVDPV-Fact-Sheet-20191115.pdf).
2
For more on VDPVs, the GPEI website offers a short explanatory video (https://polioeradication.org/polio-today/polio-
prevention/the-virus/vaccine-derived-polio-viruses).
Annex guidance
This section provides a high-level
overview on poliovirus. Further details
can be found in Annex 1. Poliovirus.
Annex guidance
For more information on VDPVs, see
Annex 2. Vaccine-derived poliovirus
classification and response.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
2
2. Polio eradication
Following the widespread use of the poliovirus vaccine in the mid-20
th
century, the worldwide incidence
of poliomyelitis declined rapidly. In 1988, the World Health Assembly adopted the goal of global polio
eradication.
The benefits of the global eradication of polio are at least threefold:
1. Reduction in morbidity and mortality: Polio is a leading cause of disability in populations not
immunized against it. With the eradication of WPV types 2 and 3 (WPV2 and WPV3), the
incidences of infection caused by these two agents have already been reduced to zero, in
addition to preventing millions of disability-adjusted life years (DALYs).
2. Strengthened health systems: The polio eradication programme has enhanced the
collaboration between the surveillance systems and laboratory networks. It has helped
revitalize immunization programmes and it contributes to the strengthening of health system
planning, management and evaluation.
3. Economic impact: It is estimated that US$1.5 billion will be saved per year after the final
remaining serotype (WPV1) is eradicated and immunization against it stopped.
Polio can be eradicated because of the following characteristics:
• poliovirus has no animal reservoir;
• poliovirus survives for a limited amount of time in the environment; and
• inexpensive and effective vaccines exist to protect the population from the disease.
More than 200 countries and territories have eliminated polio through time-tested strategies by:
• attaining high essential immunization coverage (>90%) with at least three (3) doses of polio
vaccine within the first year of life;
• conducting high-quality supplementary immunization activities (SIAs) to stop outbreaks and
interrupt the spread of the virus; and
• implementing a sensitive surveillance system for poliovirus.
The following criteria will be applied for certification of WPV eradication:
3
• no WPV transmission detected from any population source for a period of no less than two (2)
years,
• adequate global poliovirus surveillance; and
• safe and secure containment of all WPVs retained in facilities, such as laboratories and vaccine
manufacturing facilities.
Global polio-free certification will be further sustained by requirements for containment of all
polioviruses and the cessation of OPV immunization to mitigate the risk of re-emergence over time.
4
3
Global Commission for the Certification of the Eradication of Poliomyelitis (GCC). Report from the 22nd meeting of the Global
Commission for Certification of Poliomyelitis Eradication, 28-29 June 2022. Geneva: World Health Organization; 2022
(https://polioeradication.org/wp-content/uploads/2022/09/22nd-GCC-report-20220907.pdf).
4
For more on sustaining a polio-free world after the certification of global eradication, see: Global Polio Eradication Initiative
(GPEI). Polio Post-Certification Strategy. Geneva: World Health Organization; 2018 (http://polioeradication.org/wp-
content/uploads/2018/04/polio-post-certification-strategy-20180424-2.pdf). Revision in development.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
3
3. Polio surveillance systems
Different types of surveillance systems for detecting the transmission of poliovirus are critical to reach
global polio eradication, as high-quality surveillance permits the timely detection of poliovirus
transmission due to WPV, VDPVs and the circulation of Sabin-like (SL) viruses.
5
1. Acute flaccid paralysis (AFP) surveillance: This globally accepted case-based syndromic
surveillance for AFP cases confirms poliovirus by testing stool specimens in polio laboratories.
AFP surveillance remains one of the cornerstones of the polio eradication effort.
2. Environmental surveillance (ES): AFP surveillance is complemented by environmental
surveillance (ES) which systematically tests sewage samples for poliovirus in specific settings.
6
3. Immunodeficiency-associated vaccine-derived poliovirus (iVDPV) surveillance: AFP
surveillance is also complemented by surveillance for iVDPVs among patients with primary
immunodeficiency disorders (PIDs), which is referred to as iVDPV surveillance.
7
These three components of polio surveillance are supported by the Global Polio Laboratory Network
(GPLN) for confirmatory testing using viral isolation, intratypic differentiation and genomic sequencing
procedures. Ready access to data from various sources that include AFP surveillance, ES, and
laboratory surveillance are supported by a comprehensive polio information system (POLIS).
Challenges to AFP surveillance in the last mile to eradication
Challenges faced by the polio eradication programme have evolved over the years. Currently, the
main challenges that affect the quality and sensitivity of AFP surveillance are attributable to a
range of factors.
• Many countries face gaps in AFP surveillance at subnational levels, especially where
surveillance coverage may be limited for reasons such as an inability to routinely access
special populations or hard-to-reach areas.
• Delays in specimen or sample shipment to WHO-accredited laboratories can result in late
confirmation of polio cases and consequent delayed outbreak response, thereby giving
poliovirus ample opportunity to spread.
• Missed opportunities for action due to the underutilization of surveillance data can create gaps
where the virus can spread before detection and response.
• Attrition, rapid staff turnover and insufficient refresher trainings affect the quality of field and
laboratory surveillance work through the loss of institutional memory, skills and competencies.
Churn within surveillance teams also affects supervision and monitoring.
• A deprioritization of polio activities and deterioration of surveillance quality and sensitivity in
countries that have been polio-free for years creates delays in detecting importations or
emergences of poliovirus, which in turn affects the promptness and effectiveness of outbreak
response.
• Across all countries, the COVID-19 pandemic (caused by the SARS-CoV-2 virus) has also
negatively affected the sensitivity and timeliness of the AFP surveillance and laboratory
systems, even as the polio surveillance network itself lent crucial support to help contain
COVID-19, demonstrating it can go beyond polio surveillance to track vaccine-preventable
diseases (VPDs), emerging diseases, outbreaks or other major health events.*
*See Contributions of the polio network to the COVID-19 response: turning the challenge into an opportunity for polio transition.
Geneva: World Health Organization; 2020 (https://www.who.int/publications/i/item/contributions-of-the-polio-network-to-the-covid-
19-response-turning-the-challenge-into-an-opportunity-for-polio-transition).
5
Some countries also use enterovirus surveillance for the purpose of certification.
6
World Health Organization (WHO). Guidelines for environmental surveillance of poliovirus circulation. Geneva: World Health
Organization; 2003 (https://apps.who.int/iris/handle/10665/67854).
7
Global Polio Eradication Initiative (GPEI). Guidelines for Implementing Poliovirus Surveillance among Patients with Primary
Immunodeficiency Disorders (PIDs), revised 2022. Geneva: World Health Organization; 2022 (https://polioeradication.org/wp-
content/uploads/2022/06/Guidelines-for-Implementing-PID-Suveillance_EN.pdf).

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
4
PRINCIPLES of AFP surveillance
Acute flaccid paralysis (AFP) surveillance is a case-based syndromic surveillance system that has been
standardized throughout the world. The same tools, indicators and reporting systems are used in every
country. This standardized system has strengthened collaboration with immunization partners by
sharing uniform data on a weekly basis and advocating for action and support where risks and
weaknesses emerge.
A surveillance system that is specific to poliovirus is important because the characteristics of the
disease make it particularly challenging to detect:
• Only 1 in 200 wild poliovirus (WPV) infections of non-immune people results in paralysis. The
great majority of poliovirus infections are therefore “silent” as they do not cause paralysis.
• Even if a poliovirus infection causes paralysis, the clinical presentation of paralytic polio is
similar to that of other conditions, such as Guillain-Barré syndrome (GBS).
To overcome these challenges, two key measures were universally agreed to in the 1980s to improve
the sensitivity of the surveillance system:
1. adopting the syndrome of AFP as a reportable condition, and
2. laboratory confirmation of poliovirus by testing stool specimens in polio laboratories accredited
by the World Health Organization (WHO).
1. Adopting AFP as a reportable syndrome
When the Global Polio Eradication Initiative (GPEI) was first established, most countries were reporting
clinically confirmed polio cases. Polio was reported as just one of many diseases within disease
surveillance systems, often on an annual basis. Given the epidemiology and characteristics of polio, this
made it difficult to detect new cases and respond to outbreaks of polio both swiftly and effectively.
Many diseases may initially look like polio. A more sensitive
system was therefore needed to enable suspected new cases
to be detected, reported and investigated as rapidly as possible.
This led to the adoption of acute flaccid paralysis or
AFP as the syndrome to be reported.
8
This sensitive case-based syndromic definition captures not
only acute poliomyelitis but also other diseases that present
similarly, including GBS, transverse myelitis and traumatic
neuritis, such that each case must be investigated with
laboratory tests to confirm their causes. (Annex 1. Poliovirus
offers differential diagnoses and the clinical signs and symptoms which are used to differentiate
poliomyelitis from other diseases: asymmetric flaccid paralysis, fever at onset, rapid progression of
paralysis, residual paralysis after 60 days and preservation of sensory nerve function).
The rate of non-polio AFP case detection is a key indicator of AFP surveillance sensitivity. In the
absence of polio circulation, a sensitive surveillance system will detect at least one (1) case of non-polio
AFP each year for every 100 000 children under 15 years. Where polio is present or where polio is a
threat, this target is modified. The objective is then to detect at least two (2) cases of non-polio AFP
each year for every 100 000 children under 15 years in all at-risk and outbreak countries, and to detect
at least three (3) cases of non-polio AFP each year for every 100 000 children under 15 years in
endemic countries and outbreak-affected areas. (See Annex 3. Indicators for AFP surveillance.)
8
In the same way, smallpox eradication adopted detection and investigation of the “rash and fever” syndrome.
Defining AFP
An AFP case is defined as a child
under 15 years presenting with
sudden onset of floppy paralysis or
muscle weakness due to any
cause, or any person of any age
with paralytic illness if poliomyelitis
is suspected by a clinician.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
5
2. Testing all stool specimens in a WHO-accredited polio laboratory
Polioviruses are primarily transmitted from person-to-person through the faecal-oral route in settings
with poor water, sanitation and hygiene. They replicate in the human intestinal system, where they are
shed intermittently in the stool of infected individuals. Shedding is most intense up to two weeks after
the onset of paralysis but can continue up
to six to eight weeks after onset.
Collecting two (2) stool specimens,
24 hours apart from each AFP case and
within 14 days of the onset of paralysis,
and then testing them in a WHO-
accredited polio laboratory is the most
reliable way to confirm the presence or
absence of poliovirus in the specimen and
thus to confirm poliovirus infection.
One of the universally accepted indications
that an AFP surveillance system is
sensitive enough to detect poliovirus is
that at least 80% of reported AFP cases
have had their stool specimens collected
adequately. The percentage of AFP cases
with adequate stools is used as the
second key indicator of AFP surveillance
sensitivity. (See Annex 3. Indicators for
AFP surveillance.)
Gold standard indicators for AFP surveillance
Non-polio AFP rate
✓ At least one (1) non-polio AFP case each year for
every 100 000 children aged under 15 years.
✓ In endemic countries and outbreak-affected areas,
at least three (3) non-polio AFP cases each year
for every 100 000 children under 15 years.
✓ In at-risk and outbreak countries, at least two (2)
non-polio AFP cases each year for every 100 000
children under 15 years.
Adequate stool specimen rate
✓ At least 80% of reported AFP cases have had
their stool specimens collected adequately.
See Annex 3 for core and non-core
AFP surveillance indicators.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
6
STRATEGIES for AFP surveillance
Acute flaccid paralysis (AFP) cases are detected using three main strategies: routine (or passive)
surveillance, active surveillance (AS), and community-based surveillance (CBS).
9
Some supplemental
strategies for special populations and particular contexts also support overall AFP surveillance.
1. Routine (passive) surveillance
1.1 – What is routine (passive) surveillance?
The regular reporting of diseases or conditions of interest from reporting sites, such as health facilities
and hospitals, is called routine surveillance. It is sometimes referred to as passive surveillance because
public health authorities must rely on thousands of designated focal points from a variety of reporting
sites to detect and notify (or report) cases. It is also sometimes referred to as zero reporting as
reporting sites must report weekly, even
if no case has been detected.
In a majority of countries, routine AFP
surveillance is conducted as part of an
existing overall notifiable disease
reporting system that collects reports on
cases of a group of diseases or
conditions.
1.2 – AFP as a notifiable condition
Under routine surveillance, focal points at reporting sites are required to immediately report any AFP
case (i.e., within 24 hours) to a designated public health surveillance team for rapid investigation.
In addition to the immediate notification, surveillance focal points at reporting sites must also submit a
routine weekly or monthly report that must include “zero” ("0") if no AFP cases were seen in their site,
hence zero reporting. AFP is a rare condition, and a zero report is an important way to keep reporting
sites sensitized about the need to routinely conduct AFP surveillance.
1.3 – Monitoring routine surveillance
All countries are required to monitor the completeness and timeliness of routine AFP reporting, which
allows for the timely detection of gaps in reporting and surveillance quality. For most countries,
monitoring routine surveillance will be the same as the completeness and timeliness of notifiable
diseases reporting, as AFP is included among the list of notifiable diseases. These reports are also
submitted to and regularly scrutinized by National Certification Committees (NCCs) and Regional
Certification Commissions for the Eradication of Poliomyelitis (RCCs).
The indicators to monitor routine surveillance for AFP at the national and subnational level are:
• the percentage of designated sites submitting weekly reports (or “zero reports”), even in the
absence of cases, for a given time period (completeness); and
• the percentage of designated sites submitting weekly reports (or “zero reports”) on time, even in
the absence of cases, by the deadline (timeliness).
Surveillance teams should use this data to identify and follow up on reporting sites repeatedly failing to
report or reporting late. (See Annex 3. Indicators).
9
The PH101 Series by the U.S. Centers for Disease Control and Prevention (CDC) provides an introduction to public health
surveillance (https://www.cdc.gov/training/publichealth101/surveillance.html). See also Losos JZ. Routine and sentinel
surveillance models. East. Mediterr. Health. J 1996;2(1):46-50 (http://www.emro.who.int/emhj-volume-2-1996/volume-2-issue-
1/article6.html).
Defining routine surveillance
Also called passive surveillance or zero reporting, routine
surveillance is a process in which reporting sites are
expected to send reports to public health authorities
regularly and often weekly, regardless of whether an AFP
case has been seen.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
7
1.4 – Challenges with routine surveillance
The following challenges can be encountered with routine surveillance.
● Incomplete reporting networks may lead to delays in detection when the network is not
comprehensive enough (i.e., no sites in certain parts of the country).
● Incomplete weekly reports may occur when sites do not report as required, and the field team
has limited capacity either to follow up with “silent” reporting sites or to conduct training and
sensitization activities for all reporting sites. In these cases, active surveillance (below) provides
opportunities to strengthen routine surveillance through visits with site focal points.
● Attrition among personnel at the reporting site may lead to a lack of awareness of AFP as a
notifiable condition and a subsequent failure to identify and immediately report AFP cases.
● Declining awareness about polio and AFP reporting requirements may also create
confusion. Providers may forget the importance of reporting AFP as a syndrome as separate
and distinct from reporting polio as a diagnosis.
● Confusion between routine and active surveillance may lead to insufficient engagement of
both the formal and informal health sector. Under routine surveillance, district and provincial
surveillance teams rely on formal health sector sites to report on AFP cases; under active
surveillance, however, district and provincial
surveillance teams are actively engaged in finding
AFP cases by visiting health sites on a regular basis.
(In some settings, inquiries about AFP cases within a
routine reporting site made by the site-level focal point
are mistakenly considered “active surveillance.” Such
inquiries must be made by personnel external to the
facility to be considered active surveillance.)
2. Active surveillance
2.1 – What is active surveillance (AS)?
In countries and areas where people may not have access to health facilities, well-implemented active
surveillance (AS) has proven to be the most effective strategy for AFP surveillance.
Under AS, trained public health surveillance
staff regularly visit priority reporting sites
within the formal health sector (such as
tertiary hospitals and district hospitals) and
informal health sector (such as community
health centres run by nongovernmental
organizations [NGOs]) to identify and
investigate any unreported AFP cases and to
regularly sensitize targeted staff on polio and
AFP surveillance. To be effective, AS visits
must be done by well-qualified staff who
understand the polio eradication programme
and have good interpersonal skills.
Experience has shown that some countries have effectively used AS for AFP as
a platform for surveillance for vaccine-preventable diseases (VPDs) or other
outbreak-prone diseases.
Download Best Practices in Active Surveillance for Polio Eradication.
Annex guidance
For more on the differences between
routine and active surveillance, see
Annex 4. Routine and active
surveillance.
Defining active surveillance
AS is a process in which designated surveillance staff
make regular visits to the health facility; Surveillance
staff are external to the health facility. They collect
data from individual cases, registers, medical records
or logbooks at a health facility or reporting site to
ensure that no AFP case is missed.
For more on the difference between active and routine
surveillance, see Annex 4.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
8
2.2 – Setting up active surveillance
The key components of setting up an AS network are: (1) selecting, prioritizing, reviewing and updating
sites, (2) identifying focal points and building skilled surveillance staff capacity to carry out AS activities,
and (3) following a structured procedure to ensure high-quality visits.
2.2.1 – Site selection, prioritization, and updating
Selection: AS sites are drawn from the formal health sector and are a subset of the routine reporting
sites; however, they may also include some components of the informal health sector, such as
traditional health healers. In certain contexts, NGO-run facilities can also be included in the AS network,
such as where health facilities are set up in camps for refugees or internally displaced populations
(IDPs).
An analysis of where AFP reports originate will
show that the majority of children with AFP are
detected at and reported from a relatively small
number of reporting sites that are medium to
large hospitals, often referred to as secondary
or tertiary hospitals. The rationale behind this
trend is that, when faced with a health
emergency such as the sudden onset of
paralysis in a child, parents and caregivers are more likely to go to the largest accessible hospital,
bypassing local health centres and smaller hospitals.
Therefore, the primary criteria for selecting AS sites should be:
• the probability that children under 15 years of age with AFP are seen at the facility.
Additionally, AS sites should also be selected to ensure:
• the AS network is demographically and geographically well-distributed and representative of the
population in a province or district; and
• facilities within the network represent all sectors of the health system, from public and private
hospitals, to clinics and health centres, to pharmacies and even traditional healers, religious
leaders or other local community resources.
The informal health sector plays an important role, especially in locations where it represents the first
point of contact for families and communities to seek health care or advice. Informal health workers,
such as traditional medicine practitioners and faith healers who are likely to see AFP cases but do not
work within the formal health system are thus identified and sensitized to the importance of AFP and
oriented on its detection. They are then asked to contact surveillance staff upon encountering a
suspected AFP case.
Prioritization: Once all AS sites are selected, a prioritization scheme of high-, medium-, and low-
priority sites must be applied to determine the frequency with which district and provincial surveillance
staff will conduct AS visits (Table 1). The frequency of site visits depends on the priority of the facility.
The highest priority should be given to those sites that see the most AFP cases, typically larger health
facilities and hospitals. Countries experiencing an outbreak may consider adding a fourth category
(“very high-priority sites”) under which targeted facilities are visited twice weekly. Annex 5 details
processes and procedures for AS surveillance visits.
Selecting active surveillance sites
The primary criteria for selecting health facilities for
the AS network is the probability that children under
15 years of age with AFP are seen at the facility.
AS networks include reporting sites from the formal
and the informal health sector.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
9
Table 1. Site prioritization scheme
Classification
Frequency of site visits
Very high-
priority sites
Very large national referral hospitals (in countries
experiencing an outbreak)
Visited twice weekly
High-priority
sites
Very large national referral hospitals (in some countries)
Visited more than once a week
All tertiary and secondary public and private hospitals
and all hospitals with paediatric departments
Visited weekly
Medium-
priority sites
Medium-sized hospitals, smaller hospitals and large
health centres (in some countries)
Traditional healers renowned for treating paralysis (in
certain communities)
Visited every two weeks
Low-priority
sites
Health posts, small health facilities, traditional healers,
pharmacies that could see an AFP case
Visited monthly
Not prioritized
Not part of the AS network, but part of the routine
surveillance network
No AS visits for AFP surveillance
AFP = acute flaccid paralysis; AS = active surveillance
Experience in polio-endemic countries has shown that, provided the prioritization
exercise is executed appropriately, the number of sites in the high-priority group
should be lowest (10–15% of the total number of AS sites), with more in the medium-
priority group (25–35%), and the remainder of sites in the low-priority group.
Updating the AS network: National, provincial and district surveillance teams should review the AS
network twice per year and make adjustments, as needed. Facilities may have closed, or new facilities
opened. In many countries, the private health sector is growing rapidly, and new facilities may be
predominantly in the private sector. Sites should be dropped from or added to the network accordingly.
Adjusting the AS site network is especially important in conflict settings, as conflict and insecurity may
disrupt the healthcare system. In such cases, public health surveillance teams need to respond by
updating and possibly expanding the AS network in those parts of the country around inaccessible
areas and in host communities receiving IDPs
or refugees, based on their health-seeking
behaviour. Where people no longer have
regular access to health facilities, surveillance
activities should be expanded to include direct
reporting from affected communities by
including IDP and refugee camps or NGOs
that provide health services (see also
Community-based surveillance and Annex 6).
2.2.2 – Site focal points and surveillance officers
Depending on a country’s size, district, provincial or national surveillance health officers will be
responsible for organizing and scheduling regular AS visits to reporting sites in their area.
In each AS site, a suitable AFP surveillance focal point must be identified or designated if not already in
place. While different groups may be considered for this function, depending on the size of the health
facility, priority should always be given to a paediatrician, if available.
Reviewing and adjusting sites
The AS network must be reviewed and updated twice
a year to account for the opening and closing of
health facilities, as well as sociodemographic
changes to the population.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
10
The AS focal point has several key roles and responsibilities that include:
• immediately notifying an identified AFP case and providing case investigation support;
• coordinating with public health staff during AS visits; and
• confirming zero reporting for routine (passive) surveillance for formal health facilities.
In the informal health sector, such as facilities held by traditional healers or private pharmacies, the
focal point by default will be the service provider, whose responsibility will be to notify immediately any
new AFP case. These establishments are typically not part of the routine surveillance system, hence
are not expected to provide reports.
2.2.3 – Site visit procedures
At the district or provincial level, public health surveillance officers will coordinate to conduct AS visits
according to the site visit calendar and prioritization scheme (Table 1 above).
Key activities for site visits
1. Meet with the facility AFP surveillance focal point to ask
whether any AFP cases were seen and provide
surveillance and polio eradication updates.
2. Visit all relevant departments and wards and review
patient registers.
• Look for missed or unreported AFP cases since the
date of the last visit. Look for “AFP” or associated
signs, symptoms, or diagnostics (Table 2). Because
AFP surveillance is a syndromic-based surveillance, it
is important to review symptoms, not diagnoses.
• Highlight directly in the register (with a coloured marker, if possible) and crosscheck the line
listing of all AFP cases (or possible AFP cases) which were found in the register.
• Date and sign all patient registers that were reviewed.
3. Follow up on any unreported AFP cases.
• If AFP cases were already reported and investigations launched, no further action is needed.
• If AFP cases were not reported, request medical records to search for details. Visit patients in
the hospital if still admitted; if discharged, obtain addresses to visit patients at home. If the
suspected case is confirmed as AFP, conduct the AFP case investigation and initiate specimen
collection (see Case investigation and validation under Case activities for AFP
surveillance, as well as Annex 8). In addition, speak to the physician or nursing staff to inquire
why the case was not reported and sensitize them to report such cases immediately. Conduct
follow-up visits to ensure that no additional AFP cases are missed and that all relevant staff has
been sensitized.
4. In addition, assess the overall status of polio-related functions during the visit.
• Take opportunities to sensitize department and ward staff on polio and AFP surveillance.
• Determine whether and when a training session may be needed, such as after staff turnover.
Experience has shown that, particularly in larger university hospitals, AS is more
efficient when performed by senior staff who have experience working with clinicians.
They can be shadowed by junior staff, who will in turn learn to build rapport with
clinicians and eventually conduct AS visits independently.
Annex guidance
Surveillance officers should always
follow standard procedures to
structure AS visits. See Annex 5.
Active surveillance visits and
Annex 7 for an example of an AS
visit form to support data collection
and monitoring.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
11
• Ensure sufficient supplies and resources are available, including forms, stool kits, and posters.
• Check immunization-related equipment and supplies, such as vaccines (oral polio vaccines
[OPVs] and/or inactivated polio vaccine [IPV]) and cold chain storage and carriers.
• Check into other VPD surveillance functions alongside AFP surveillance. As the integration of
AFP surveillance into VPD surveillance progresses, it is important to take advantage of AS
visits and search for and collect data on other VPDs or outbreak-prone diseases.
Table 2. Possible indications of an AFP case in patient registers
Disease conditions always
presenting as AFP
● Paralytic polio
● Guillain-Barré syndrome (GBS)
● Transverse myelitis
● Traumatic neuritis
Disease conditions which may
initially present with AFP
● Pott’s disease (spinal tuberculosis)
● Bacterial or tuberculous meningitis
● Encephalitis
● Cerebrovascular accidents (stroke)
● Hemiplegia
Other signs and history to be
considered suspicious, indicating
that AFP may have been present
initially
● Frequent falls
● Weakness, paresis
● Abnormal gait, unable to walk, difficulty in walking
● Easy fatigability
AFP = acute flaccid paralysis; GPS = Guillain-Barré syndrome
2.3 – Monitoring active surveillance
The completeness and adequacy of AS visits must be monitored at the district, provincial and national
level. For a list of indicators used to monitor AS, see Annex 3. Indicators for AFP surveillance.
Monitoring is best accomplished by using a form that is completed by the visiting surveillance officer
and submitted after each visit to a supervisor at the provincial level. Annex 7. Examples of forms
offers a sample AS visit report. The form collects key data on all AS visits: the date, time and location,
facility visited, and a list of departments visited within large hospitals, as well as whether an undetected
AFP case was found during the visit, whether any AFP sensitization or orientation activities were
conducted, and whether supplies were provided to the facility (e.g., stool collection kits or posters).
Monitoring AS visits via mobile data and visualizing the analysed data can help
identify blind spots in the surveillance network and accelerate corrective actions.
See Monitoring AFP surveillance for more innovations in disease surveillance.
2.4 – Challenges with active surveillance
As public health teams implement AS, several challenges may arise.
Insufficient resources: After establishing the reporting network, surveillance teams often report
insufficient resources (such as not enough time, qualified staff, or means of transportation) to conduct
visits to all AS sites in the network.
• If this issue occurs, it is very important to ensure that at least all high-priority sites are visited
regularly, followed by as many medium- and low-priority sites as possible. This should be
feasible as a majority of high-priority sites (e.g., large hospitals) are in national or provincial
capitals and relatively close to the national or provincial surveillance office.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
12
• For facilities that cannot be visited, facility focal points should be contacted regularly by phone
or email, in addition to monitoring routine reports from these sites.
• Lists of sites and a calendar of visits should be reviewed or re-adjusted regularly until more
resources are made available.
Lack of attention to capital cities: AFP quality indicators from national capitals and the capital regions
of many countries tend to be surprisingly low. This is difficult to account for, as these areas usually host
large university hospitals and tertiary care facilities and large numbers of AFP cases are seen in these
areas, including cases referred from the provinces. Sensitive AFP surveillance at this level is more
important than anywhere else in the country.
• Large hospitals and high-priority tertiary care should be mapped and enrolled as reporting sites,
with subsequent AS visits planned and conducted on a regular and frequent basis.
• Visits must be conducted by surveillance officers who are trained and experienced in
sensitization and who are comfortable with medical personnel. These visits should be
accompanied by supportive supervision and monitoring for timeliness and completeness.
Inexperienced staff conducting AS visits: To successfully use AS visits for continuous sensitization
of clinicians and other hospital workers on AFP surveillance concept and practices, public health
officers must be trained on establishing rapport with medical staff, including with the chiefs of units,
some of whom may still not accept or fully understand syndromic AFP surveillance.
• Country programmes should commit to building junior staff capacity through supportive
supervision. Good mentoring and training ensure staff are well-qualified and equipped with
strong interpersonal communication skills.
• Particular attention should be given to female public health officers who may encounter gender
barriers while interacting with medical and hospital administrative staff.
Lack of access at private hospitals and facilities: AS visits can be challenging in private, military or
other sector-specific facilities. Surveillance officers should be aware of this and may need support from
higher-level officials to renegotiate access at regular intervals.
Insufficient geographic and demographic coverage or representativeness: The AS network may
possess geographic or demographic blind spots. Surveillance teams should be vigilant to identify:
• overlooked population groups that live in remote or hard-to-reach areas;
• overlooked mobile populations, such as refugees and IDPs;
• overlooked informal health sector sites, including traditional medicine or faith-based healthcare
facilities, or other healthcare sites, such as military or private facilities;
• AS sites not visited for long periods;
• AS sites not updated, thus missing newer facilities or potentially key practitioners; and
• AS sites that have closed down.
Changes can only be made through regular reviews and a thorough mapping of healthcare sites.
Special populations and the health-seeking behaviour of cases and their caregivers are also needed to
identify and address potential weaknesses and gaps in the active surveillance network (see Annex 9.
Health-seeking behaviour).
In most countries, passive and active surveillance are used in parallel.
Both systems use the same network of reporting sites, but AS takes only a subset
that are further prioritized for surveillance of AFP and classified as high-, medium-,
and low-priority sites. (See Annex 4. Routine and active surveillance.)

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
13
3. Community-based surveillance
3.1 – What is community-based surveillance?
Community-based surveillance (CBS) is a surveillance strategy in which trained community members
are engaged to report suspected AFP cases to a designated focal person based on a simple AFP case
definition.
10
What distinguishes CBS from routine and
active surveillance is that case detection
occurs outside health facilities and that
those performing case detection activities
are community members, not health
professionals.
CBS is a key method to access hard-to-reach areas and communities that are not reached by the
regular AFP surveillance system (see Supplemental strategies for special populations). CBS may
be particularly useful in settings or areas at high risk of undetected poliovirus transmission or at risk of
new outbreaks following importation or vaccine-derived poliovirus (VDPV) emergence.
Settings conducive to CBS include:
• security-compromised areas;
• mobile populations such as nomads and seasonal workers;
• special populations that are underserved, such as refugees, IDPs, slum dwellers, ethnic
minorities, isolated religious communities or remote populations in hard-to-reach areas; and
• areas or populations relying largely on traditional medicine, where people are less likely to seek
care at a health facility.
CBS provides a link between communities and the AFP surveillance system through a designated focal
point – and it may increase community engagement in health care and acceptance of immunization and
surveillance activities.
While CBS can increase the sensitivity and timeliness of AFP case detection, it can also be resource-
intensive and should be used only where health facility-based surveillance cannot be performed or is
not functioning well. CBS methods range in resource intensity. Training, sensitization, and supervision
are minimum essential activities, and the addition of other activities comes with increased costs. Major
cost drivers include: training (initial training and
refreshers); supervision; reporting incentives or monthly
payment; and the use of digital technology, mobile
phones, or other tools (initial and recurring costs).
When considering CBS, countries should note that this
strategy may be more cost effective if used for multiple
diseases rather than a single disease.
3.2 – Setting up community-based surveillance
Initiating CBS should be carefully assessed because of its resource-intensive nature. Other
sensitization activities or adjustments to the AS network may be more efficient for closing surveillance
gaps. Programmes are advised to look first at more sustainable, cost-effective solutions.
A needs assessment must be conducted to first determine if CBS should be endeavoured. The needs
assessment explores key questions that include: How well does the current AFP surveillance system
cover or reach special populations or hard-to-reach areas? What are the real issues behind surveillance
10
Rather than the full standard AFP case definition (see Principles of AFP surveillance, section 2), a simplified AFP case
definition should be used when sensitizing community informants, such as: “Report all children with sudden presence of floppy
paralysis or weakness.”
Defining community-based surveillance
CBS is an AFP surveillance strategy that relies on
trained community members to identify cases in areas
and communities with limited access to health facilities.
Annex guidance
For more information, including steps
toward establishing CBS, see
Annex 6. Community-based surveillance.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
14
gaps? Are CBS activities currently operating for other diseases? See Annex 6 for more guiding
questions that can inform a CBS needs assessment.
Steps to establish CBS include the following activities:
1. Identify key community members, such as local and religious leaders.
2. Sensitize and brief them about polio and AFP; ask for their advice to select community volunteers.
3. Select and train volunteers on their role in CBS. Engage both male and female community
volunteers. Women can facilitate CBS in areas where access to female household heads or
members is not customary for men. Similarly, the presence of a female team member can facilitate
engaging with and accessing more traditional communities.
4. Link volunteers with a designated focal point and/or surveillance officer who will follow up and verify
AFP cases, investigate and initiate stool collection.
In some countries, CBS can be set up for the purpose of AFP surveillance only, while
in other countries, CBS is an already-existing network that is fully integrated in the
public health system for VPDs and outbreak-prone diseases, of which AFP
surveillance is only one part.
3.3 – Monitoring community-based surveillance
CBS should be carefully monitored, particularly for context-specific challenges such as hard-to-reach
populations and inaccessible areas.
Key indicators to monitor CBS include:
• percent of AFP cases reported by CBS compared with AFP cases notified by reporting sites in
the specific area; and
• percent of initial AFP case reports verified as “true AFP” versus “not AFP.”
Complete indicators are available in Annex 6. Community-based surveillance and Annex 3.
Indicators for AFP surveillance.
3.4 – Challenges with community-based surveillance
• Implementing and sustaining effective CBS can be resource intensive. The resources needed for
CBS depend upon the country context and the decisions of the surveillance team.
• Hard-to-reach areas present unique challenges for ensuring a reliable line of communication
between community informants and surveillance officers. To address this, some teams offer mobile
phones or dispense petty cash to pay for communication expenses.
• Low literacy levels within local communities may require more time and effort on the part of the
public health staff for adapting AFP surveillance training and sensitization protocols.
• Partially or fully inaccessible areas can impede monitoring and supportive supervision of CBS
informants, as well as create problems for conducting AFP case verification and investigation. If this
occurs, AFP cases may need to be brought outside inaccessible areas for investigation.
• A considerable percentage of reports of “suspected AFP” may not meet the standard AFP case
definition and may give a low yield of actual (“true”) AFP cases, which may increase the workload of
public health staff through the added time needed for verification and investigation.
For more on CBS-related challenges and solutions, see Annex 6.
CBS for polio can be also referred to as “a network of informants,” “village polio
volunteers” or “informers.” Depending on the country, community volunteers may or
may not be remunerated or financially motivated and may or may not be working full
time on polio surveillance.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
15
4. Supplemental strategies for special populations
Certain population groups are underserved or not served at all by health systems. They are also
persistently missed by surveillance efforts. While the reasons for these gaps are varied, one finding is
that persistently missed population groups often belong to high-risk mobile populations or reside in
hard-to-reach or inaccessible areas, including areas affected by insecurity and conflict. These special
population groups are particularly important for disease control and eradication programmes because
they have higher susceptibility to infection due to low immunization coverage and are therefore more
likely to transmit viruses – and more likely to be missed by surveillance systems.
Guidelines for Implementing Polio Surveillance in Hard-to-Reach Areas and Populations details some
strategies (of which CBS is one approach) for implementing surveillance among special populations,
with a focus on high-risk mobile populations.
11
4.1 – What are special populations?
Several different marginalized population groups are at
risk of being underserved or altogether missed by
surveillance efforts. These include:
● mobile populations: nomads and seasonal migrants
such as agricultural, mine, brick kiln or construction
workers;
● refugees and IDPs living in camps and in host
communities;
● populations in settled areas which are underserved
by existing health services such as cross-border
populations, slum dwellers, ethnic minorities,
islanders, fishermen and those living in hard-to-
reach areas; and
● totally inaccessible population groups, such as
those in security-compromised and conflict-affected
areas.
4.2 – Identifying and mapping special groups
By identifying, mapping and profiling unserved or underserved populations, special surveillance
strategies can ensure that such populations are covered by polio immunization and surveillance.
The following data and information are critical to better characterize and reach such groups:
● geographic location and population size for mobile groups: itineraries and routes of migration,
timing and possible seasonality of nomadic movement;
● current access to health services and health-seeking behaviour (see Annex 9. Health-seeking
behaviour);
● availability of the existing surveillance network (facility- or community-based) to detect AFP cases in
this special population;
● identification of service providers who exist in the area but are not yet participating in polio activities
(public and private, including NGOs or faith-based organizations);
● availability of options to develop communication activities targeting these special groups;
● means of communication through the availability of network coverage and/or readily available use
of cell phones for public health officers and community workers and volunteers; and
● general information, such as language, literacy, community structure in terms of leaders and
influencers.
11
Global Polio Eradication Initiative (GPEI). Guidelines for Implementing Polio Surveillance in Hard-to-Reach Areas &
Populations. Geneva: World Health Organization; 2017 (https://polioeradication.org/wp-content/uploads/2020/10/Guidelines-polio-
surveillance-H2R-areas.pdf).
Special populations and insecurity
While some countries have hard-to-reach
areas due to geographic barriers and
transportation issues, some countries face
particular challenges in insecure and
conflict-affected areas.
In Borno State, Nigeria, ongoing conflict and
insecurity left large parts of the population
inaccessible for a prolonged period of time,
resulting in WPV1 transmission missed for
several years and detected late in 2016.
Parts of Syria, Yemen and Iraq have
historically faced similar scenarios, where a
lack of security and safety prevents field
staff from reaching communities to conduct
immunization and surveillance activities.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
16
4.3 – Implementing a mix of surveillance strategies for each special group
Once special populations have been identified and
profiled, surveillance approaches can be specifically
tailored to ensure each group is adequately covered by
poliovirus surveillance (Table 3). A set or mix of
suggested surveillance strategies for each kind of special
population is recommended.
12
The key recommended strategies are:
1. Enhanced AFP surveillance with ad hoc AFP case search and systematic contact sampling.
• Ad hoc AFP case search in large gatherings of nomads, for example during SIAs and
during mobile outreach services (Annex 11).
• Systematic AFP contact sampling for all inadequate AFP samples, with one sample each
from three contacts of an AFP cases with inadequate samples, for example. However, in
coordination with surveillance and laboratory teams, this can be expanded to all AFP cases
from special populations (Annex 12).
2. Targeted healthy children sampling can be conducted in special populations that are at high
risk for poliovirus; however, this is not a routine strategy and can only be initiated in
coordination with and with the approval of surveillance and laboratory teams at the national and
regional levels (Annex 13).
3. Ad hoc environmental surveillance sampling sites can enhance surveillance in areas
considered at high risk of poliovirus circulation because of an outbreak or the sudden influx of
an at-risk population.
13
This strategy should only be considered after strengthening AFP
surveillance and in coordination with the laboratory.
Table 3. Examples of activities by type of special populations
Population type
Activity examples
Populations living
in security-
compromised
areas
● Access mapping and analysis of population dynamics and movements; access
negotiation, if needed.
● Coordination with armed forces or groups and relevant partners.
● Review of surveillance network and establishment of CBS as appropriate, including
identifying and training appropriate focal points.
● Enhanced surveillance in parts of the country bordering inaccessible areas and
wherever IDPs come out of inaccessible areas and are received (e.g., adding to
reporting sites based on health-seeking behaviour, identification and training of local
informants).
CBS = community-based surveillance; IDP = internally displaced populations
12
Global Polio Eradication Initiative (GPEI). Guidelines for Implementing Polio Surveillance in Hard-to-Reach Areas &
Populations. Geneva: World Health Organization; 2017 (https://polioeradication.org/wp-content/uploads/2020/10/Guidelines-polio-
surveillance-H2R-areas.pdf).
13
Global Polio Eradication Initiative (GPEI). Standard Operating Procedures (SOPs) for Polio Environmental Surveillance
Enhancement Following Investigation of a Poliovirus Event or Outbreak. Geneva: World Health Organization; 2020
(https://polioeradication.org/wp-content/uploads/2021/02/SOPs-for-Polio-ES-enhancement-following-outbreak-20210208.pdf).
Annex guidance
For surveillance strategies suitable to
different kinds of special populations, see
Annex 10. Special populations groups.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
17
Table 3 (continued)
Population type
Activity examples
Nomadic
populations
● Mapping and profiling of nomadic groups in coordination with nomad leaders; AFP
focal points designated for each nomad group.
● Determining itineraries and migration pathways; mapping healthcare facilities and
providers, as well as veterinary services, along the route.
● AFP sensitization among providers and in public places along migration pathways
(i.e., in markets, at watering points and camps frequented by nomads); study of
nomads’ health-seeking behaviour.
● Regular contact with AFP focal points established and maintained.
● A similar approach should be used for other mobile population groups, as appropriate:
seasonal migrants; mine, brick kiln and construction workers; etc.
Refugees and IDPs
in camps
● Camp AFP focal point identified, designated and included in the AS network.
● Profile assessed of new arrivals: origin, immunization status, etc.
● Active AFP case search.
● Permanent vaccination and surveillance team installed.
Refugees and
informal IDPs in
host communities
and outside camps
● Key informants identified from the community and included in AS network (see
Community-based surveillance).
● Tracking of IDPs and refugees in the community via special “tracker teams” to support
understanding their health-seeking behaviour.
● AS network adjusted to include providers serving refugees and IDPs.
Cross-border
groups
● Mapping of official and informal border crossings, villages and settlements, special
groups, gathering places and seasonal movements; surveillance networks installed on
both sides of the border.
● Averages estimated for numbers of population moving and migrating across borders.
● Regular contact between AFP surveillance officers on both side of the border to
ensure sharing of data, cross notification, joint investigation and tracking of mobile
groups.
● Organizations working at border entry and exit points identified (e.g., immigration, port
health services and police); orientation and sensitization on polio and AFP
surveillance provided to healthcare workers on both sides.
Communities in
urban slums
● Profile of communities and their origin.
● Health-seeking behaviour studied, with adjustments to AS network.
● Active AFP case search conducted.
● Evaluation of any need to add environmental surveillance (ES) sites.
Other hard-to-
reach communities
● Mapping and profile of special populations who may live in remote areas such as
islanders and highlanders, or ethnic minorities who may not access the same health
facilities as the broader population.
● Identification of and regular contact with local key informants.
● Study health-seeking behaviour of these communities and adjust the network.
AFP = acute flaccid paralysis; AS = active surveillance; CBS = community-based surveillance; IDP = internally displaced population
The decision to develop, implement and possibly modify any of these strategies should be discussed by
all stakeholders involved at the local, national, and regional levels, including national or regional
laboratories.
4.4 – Challenges with supplemental strategies for special populations
Challenges to anticipate when implementing poliovirus surveillance in special groups are similar to
those listed for CBS. See also Annex 10. Special population groups and Annex 6. Community-
based surveillance.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
18
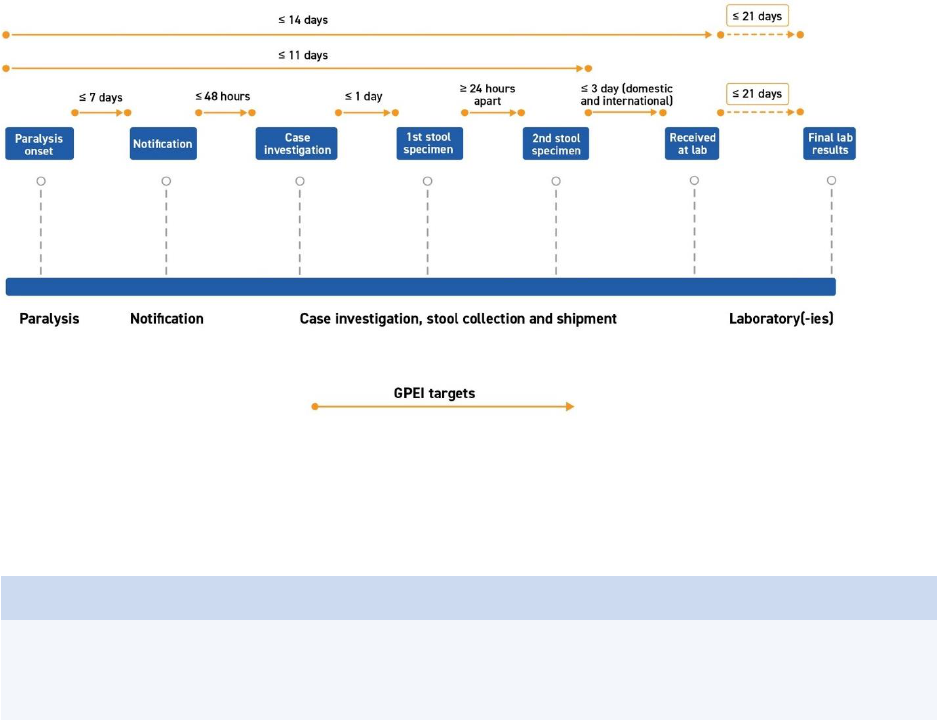
CASE ACTIVITIES for AFP surveillance
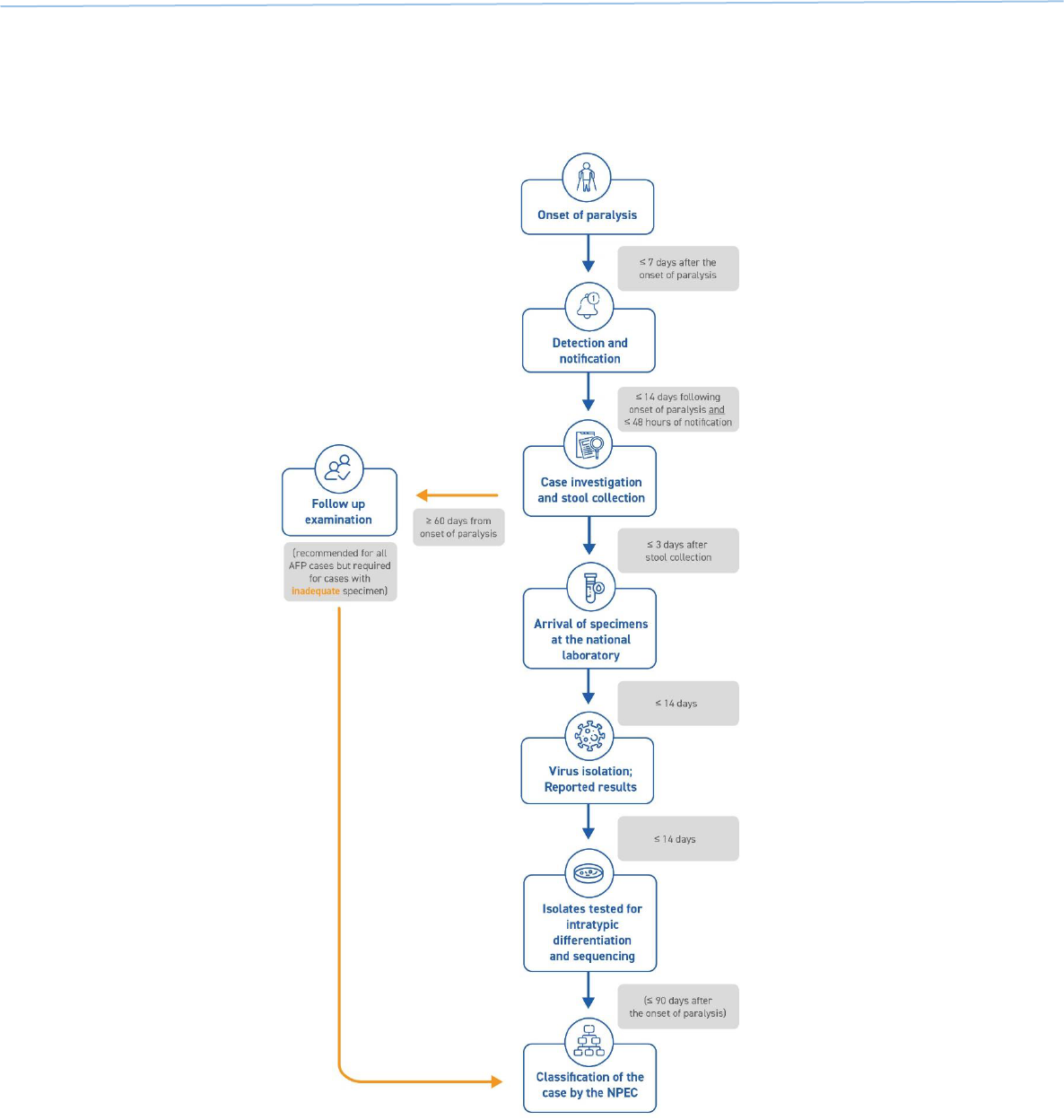
Case-related activities for acute flaccid paralysis (AFP) surveillance – from the onset of paralysis in a
patient to final case classification – require timely coordination between field and laboratory surveillance
(Fig. 1). All case-related activities should progress quickly so final classification by a National Polio
Expert Committee (NPEC) takes place within 90 days of paralysis onset.
Fig. 1. Process of AFP surveillance
NPEC = National Polio Expert Committee
Source: WHO.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
19
1. Timely detection
Responding swiftly to a possible case is critical: the earlier poliovirus is detected and confirmed, the
faster outbreak response can be implemented to end transmission. The goal established by the Global
Polio Eradication Initiative (GPEI) under its 2022–2026 Strategy is that all polioviruses should be
confirmed and sequenced within 35 days of the onset of paralysis.
14
Given this accelerated timeline,
field and logistics activities – from onset of paralysis to the arrival of stool specimens at a WHO-
accredited polio laboratory – must be completed within 14 days (Fig. 2). Note that certification
standards and indicators remain unchanged (see Annex 3. Indicators for AFP surveillance).
Fig. 2. Timeliness of detection, 35 days (onset to final lab result)
Source: WHO.
1.1 - Reduce delays
Every stage of the process depicted above (Fig. 2) should be targeted for time-saving interventions, as
timeliness will be closely monitored (see Monitoring and Annex 3. Indicators for AFP surveillance).
2. Case notification and verification
To support case verification and investigation, all supplies and materials should be prepared in advance
to allow quick deployment of the investigation team. This includes case investigation forms (CIFs),
laboratory request forms, stool specimen collection kits and stool carriers.
2.1 – Notify the case
AFP cases must be notified within seven (7) days of the onset of paralysis. A physician, health worker,
or community informant or volunteer who identifies an AFP case must report it immediately to (or
notify) the public health surveillance team at the district or provincial level.
When in doubt, always report and investigate.
14
Global Polio Eradication Initiative (GPEI). Polio Eradication Strategy 2022–2026: Delivering on a promise. Geneva: World
Health Organization; 2021 (https://apps.who.int/iris/bitstream/handle/10665/345967/9789240031937-eng.pdf).
Annex guidance
Annex 14. Rapid case and virus detection highlights bottlenecks and delays that may occur at various
stages and administrative levels, their possible causes, and ways the programme can address them.
Definitions to support case investigations are found in Annex 1. Poliovirus.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
20
3. Case investigation and validation
Once reported, the case must be investigated within 48 hours of notification by a trained,
designated AFP focal point or surveillance officer who completes the CIF.
To minimize the risk of missing key information that may explain delays in detection, CIFs capture the
social profile of cases and their community, as well as health-seeking behaviour and gender-related
information. (See Annex 7. Examples of forms for modified CIFs for endemic and non-endemic
countries.)
3.1 – Verify the case
Before starting the investigation, the AFP focal point or surveillance officer must verify whether the case
meets the AFP case definition. An AFP case is defined as a child younger than 15 years of age
presenting with sudden onset of floppy paralysis or muscle weakness due to any cause, or any person
of any age with paralytic illness if poliomyelitis is suspected by a clinician.
Verification ensures cases are systematically prepared for review and investigation.
• If the case meets the case definition, the investigation is carried out.
• If the case does not meet the case definition, the AFP focal point/surveillance officer stops the
investigation and records the case as ‘not an AFP’ on the CIF. The reasons for which the case
was verified as ‘not an AFP’ should be clearly documented. A list of these cases should be kept
separately.
• If the case has died, the investigation still needs to be conducted. The CIF must be filled with
the case history (date of paralysis onset; travel history of the case; history of health seeking;
household members and visitors) and AFP contact specimen collected. (See Section 5. AFP
contact sampling and Annex 12). Such cases will be sent to the NPEC for classification.
• If in doubt as to whether the case meets the definition or not, the case should be investigated.
For this step, verification does not require filling out any separate forms, and the verification is not
recorded as an activity in any line list.
3.2 – Investigate the case
Within 48 hours of the notification, the surveillance officer proceeds to investigate the case by
performing the following steps.
1. Speak to the physician or health worker who
reported the case and inquire about the working
or provisional diagnosis currently being
considered if the case was seen by a physician.
(See “Differential diagnosis” under Annex 1.
Poliomyelitis. Signs and symptoms to look out
for are asymmetric flaccid paralysis, fever at
onset, rapid progression of paralysis and the
preservation of sensory nerve function.)
2. Invite the attending physician or health worker to join in the case investigation.
3. Document the case by taking the patient’s history from the caregiver, transcribing it to the CIF,
including both the travel history and past history of healthcare-seeking contacts.
4. Conduct a physical examination. Note that the objective of the clinical examination in AFP case
investigation is to establish whether there is any degree of paralysis or paresis or not,
regardless of the current clinical diagnosis. It is therefore NOT to establish an exact medical-
neurological diagnosis.
5. Begin to organize the collection of two stool specimens.
Annex guidance
For a detailed explanation of how to
conduct the investigation of an AFP case
(case documentation, history taking,
physical examination and stool collection),
see Annex 8. AFP case investigation
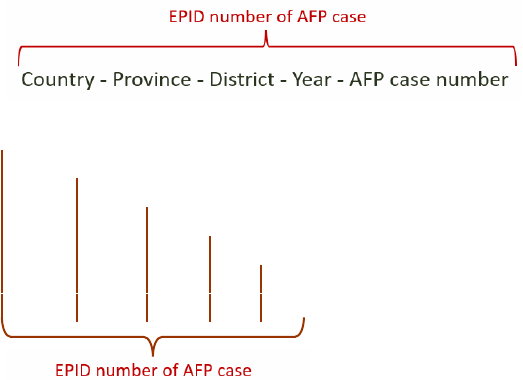
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
21
3.3 – Assign an EPID number
A unique case epidemiologic identification (EPID) number must be assigned to each AFP case. The
EPID identifies the location and year the onset of paralysis took place and indexes the AFP case count
of that location. The EPID number should be assigned at the time of case investigation so that it can be
used in the CIF and the laboratory request form. This is usually done with coordination at the provincial
or the national level, depending on the country.
The EPID number is a 14-character string that consists of the following codes (Figs. 3 and 4):
• 1
st
to 3
rd
characters specify the country code in letters
• 4
th
to 6
th
characters specify the first administrative level (usually province) in letters.
• 7
th
to 9
th
characters specify the second administrative level (usually district) in letters.
• 10
th
to 11
th
characters specify the year of paralysis onset.
• 12
th
to 14
th
characters represent the 3-digit number of the case (using a chronological order)
Fig. 3. Nomenclature for EPID
Fig. 4. Example of EPID number assignment
Country name: Newland
Province name: Province A
District name: District B
Onset year: 2022
Chronological order of case notification (i.e., 3
rd
case notified in this district in 2022)
NEW-
PRA-
DIB-
22-
03
International and national cross-notification: If it is ascertained that the onset of paralysis occurred
in a country other than where the AFP case was detected, the AFP case will be assigned (or re-
assigned) to the location where onset occurred. All parties should be informed, including field, data and
laboratory surveillance teams. International cross-notification is facilitated by the WHO regional office.
National cross-notification is usually coordinated at the subnational level, according to national
guidelines. The EPID number assigned to the case may also need to be modified accordingly.
3.4 – Validate the case
Crosschecking the accuracy of information and data recorded in the CIFs by someone other than the
person who reported the case is referred to as AFP case validation. It is ideally conducted within 14
days of the original case investigation by senior surveillance staff, typically by secondary and tertiary
supervisors, with the case and caregivers. The focus of case validation should be given to critical data:
date of onset, place of onset, areas visited prior to onset, stool collection dates/processes, vaccine
doses received by essential immunization (EI) and supplementary immunization activities (SIAs),
health-seeking history, and collection of appropriate contact samples. AFP surveillance data must be
updated based on validation findings, and discrepancies systematically recorded.
For a subset of reported AFP cases either selected at random or based on country programme-specific
criteria (such as an unexpected increase in reporting), the target for case validation is 30% measured
on a monthly or quarterly basis, depending on the country’s epidemiological status and risk.
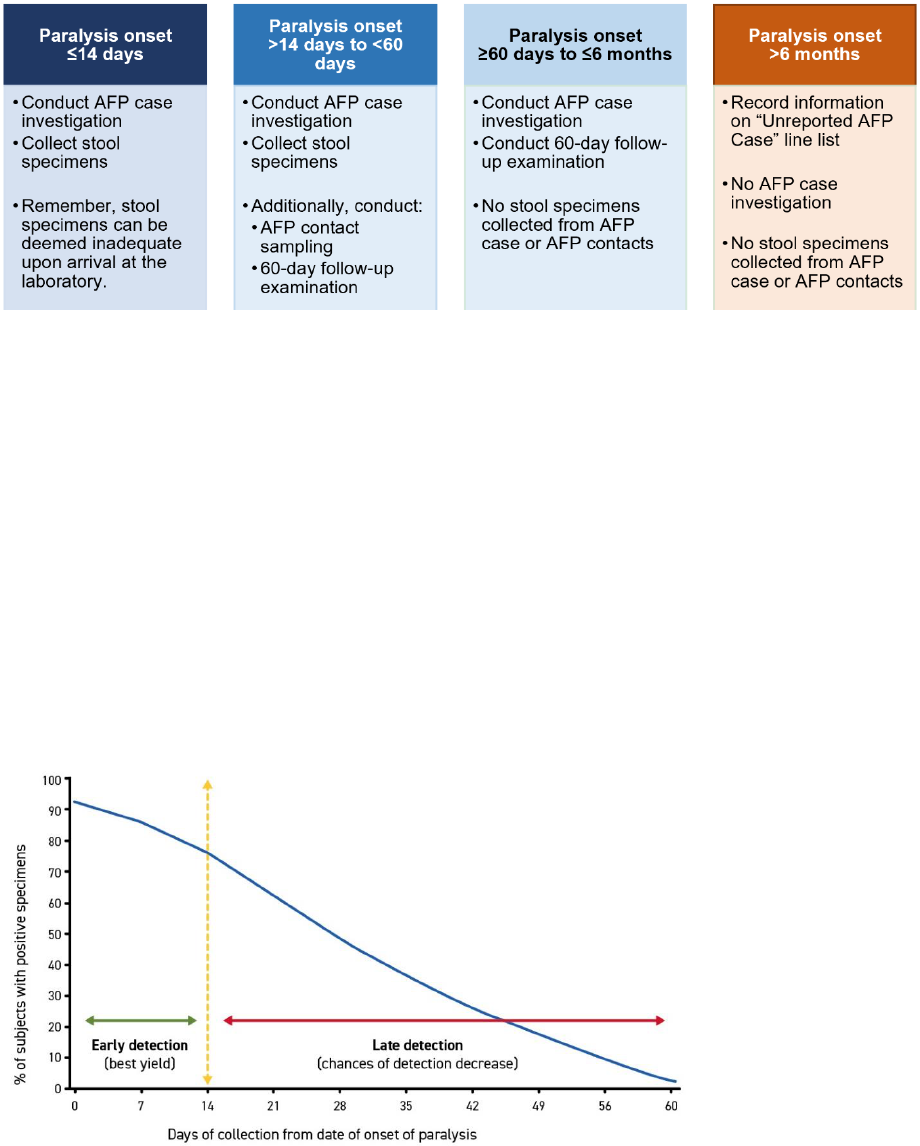
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
22
4. Stool collection and transport to the laboratory
4.1 – Collect stool specimens
To optimize isolation of poliovirus from a WHO-accredited polio laboratory, two stool specimen must be
collected as soon as possible, preferably within 14 days and no later than 60 days after the onset of
paralysis (Fig. 5).
Fig. 5. Stool collection based on onset of paralysis
AFP = acute flaccid paralysis
Source: WHO.
The chances of isolating poliovirus are greatest when the two specimens:
• are collected as soon as possible after onset of paralysis (the first specimen should therefore
be collected at the time of the investigation or as soon as possible thereafter);
• are collected within 14 days of paralysis onset and no later than 60 days;
• are collected at a minimum of 24 hours apart from each other’s collection; and
• arrive at a WHO-accredited laboratory within three (3) days of collection with good reverse cold
chain.
Virus shedding is intermittent, hence the need to collect two specimens 24 hours apart. It is also most
intense during the first two weeks after paralysis onset, hence the need to collect the two specimens as
soon as possible and no later than 60 days after paralysis onset (Fig. 6). For cases detected after 60
days after paralysis onset and up until six months after onset, a CIF should still be completed but no
stool specimens should be collected (see Fig. 5 above).
Fig. 6. Poliovirus detection in stool specimens
Source: Adapted from Alexander JP, Gary HE, Pallansch MA, Duration of Poliovirus Excretion and Its Implications for Acute Flaccid
Paralysis Surveillance: A Review of Literature, J Infect Dis 175(1):S175-82;1997 (https://doi.org/10.1093/infdis/175.Supplement_1.S176).

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
23
Stool specimens should ideally be collected at a health facility by trained personnel. If specimens
cannot be collected at a health facility and must be collected by a caregiver at the home of the case, a
sample collection and transport kit with frozen ice packs should be left with the caregivers. Ensure the
instructions are clearly understood, using simple language if needed, with contact information in case of
questions or problems arise. Make an appointment to
change melted ice packs and collect both specimens.
Annex 8. AFP case investigation provides a standardized,
step-by-step procedure for stool collection, including a list of
materials and supplies.
Stool specimen collection needs to be adequate to maximize
the laboratory’s chances of isolating and confirming the
presence of poliovirus. Inadequate collection of stool
specimens points to gaps in surveillance quality and may
lead to missed transmission of the virus.
Inadequate stool collection can be due to:
• late detection of the case (samples collected >14
days of the onset of paralysis);
• late investigation (samples collected >14 days of the
onset of paralysis);
• the death of the case or loss to follow-up of the case before sample collection;
• constipation of AFP case (i.e., zero or one stool specimen collected);
• improper collection procedure or bad conditioning (such as leaks from non-recommended
containers);
• poorly maintained reverse cold chain; and
• samples lost in transit.
The probability of not isolating poliovirus in inadequate stool specimens is high. AFP contact sampling
is therefore recommended to increase the probability of confirming polio through epidemiological
linkage for all AFP cases with inadequate stool specimens (see Section 5 and Annex 12).
4.2 – Store and transport specimens
In many countries, the WHO and the Ministries of Health (MOH) have contracts with commercial courier
companies to provide ground transport and/or air transport service to speed and/or facilitate specimen
transport. Based on established indicators, transport time from collection of the second stool specimen
to arrival to the WHO-accredited laboratory should not exceed three (3) days, irrespective of whether
the laboratory is located within the country. Specimens should be kept in a reverse cold chain at all
times.
Adequate stool collection
• Two (2) stool specimens.
• Collected at a minimum 24 hours
apart from each other’s collection.
• Collected within 14 days of the
onset of paralysis.
• Received at a WHO-accredited
laboratory in good condition
(at least 8 grams, reverse cold
chain maintained from collection to
arrival at laboratory, with no
evidence of desiccation or spillage).
Temperature effects on poliovirus
The properties of wild poliovirus type 1 (WPV1) show the risks of exposing stool specimens to prolonged
high temperatures.
• At 25°C, WPV1 is highly stable for at least 28 days
• At 35°C, WPV1 is stable for four days but becomes undetectable by 16 days.
• At 45°C, WPV1 is undetectable by day four.
The probability of detecting virus is further reduced if the concentration of irus in the specimens is low.
To be confident the virus is retained if it is present, stool specimens must be sealed in containers and
stored immediately inside a refrigerator or placed between frozen ice packs at 4-8°C in a cold box, ready
for shipment to a laboratory. Undue delays or prolonged exposure to heat on the way to the laboratory
may destroy the virus.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
24
Stool specimens should arrive at the laboratory in good condition with the following criteria met:
• adequate quantity (8–10 grams in each container, the size of two adult thumbnails);
• no leakage and no desiccation or drying out of the specimens;
• appropriate temperature and reverse cold chain maintained; and
• complete documentation (CIF and laboratory request form).
4.2.1. Maintain the reverse cold chain during storage and transport
Reverse cold chain refers to a system of storing and transporting samples at a temperature between 4°
and 8°
C from the moment of collection until arrival at the laboratory. It is critical as an interruption of the
reverse cold chain through prolonged exposure to higher temperatures or repeated freezing and
thawing may decrease the ability of the laboratory to isolate the poliovirus.
Specimens must be stored at precise temperatures determined by when they can be sent to the
laboratory (Table 4). “Batch send-off,” or delayed shipping to the laboratory until several specimens
have been collected, should be avoided as it increases the risk of interrupting the reverse cold chain
and inactivating the poliovirus so that virus detection is delayed or impossible.
Table 4. Storage requirements based on transport schedule
If transport occurs …
Storage mechanism and temperature requirement
≤72 hours after collection
Store samples in specimen carriers with frozen ice packs 4°–8° C.
>72 hours after collection
Store samples in a freezer at or below -20°C until transport to the laboratory is
ready. Do not freeze with vaccines or food.
5. AFP contact sampling
The sensitivity of an AFP surveillance system to detect ongoing circulation of WPV1 or VDPVs can be
increased by collecting and examining stool specimens from children who have been in direct contact
with the AFP case as they are likely to have a subclinical or asymptomatic infection.
AFP contact sampling is the collection and testing of one (1) stool specimen from three (3) children
who have been in direct contact with an AFP case in the week prior to the onset of paralysis and/or in
the two-week period after onset.
The recommended criteria to define AFP contacts are:
• children preferably younger than 5 years of age;
• children in contact with the AFP case within the week prior to or two weeks after onset of
paralysis; and
• children with frequent contact with the AFP case, such as siblings and other children living in
the same household and/or neighbouring children who played with the AFP case during the
period of interest (e.g., touching, sharing toys and food).
5.1 – Determine if AFP contact sampling should be conducted
Select circumstances may warrant conducting AFP contact sampling to increase the sensitivity of the
surveillance system.
• Initial case investigation: AFP contact sampling should be conducted during the initial AFP
case investigation if it is known that two stool specimens cannot be collected in a timely manner
(within 14 days of onset). The contact sampling should ideally be conducted within seven (7)
days of case notification. It can be done up to 60 days (two months) after onset of paralysis of
the AFP case, though it should be noted that the longer the wait to conduct the investigation,
the lower the probability of detecting virus (if present) in the stool specimens.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
25
• Follow-up based on inadequate stool specimens: AFP contact sampling should be
conducted if the laboratory reports the stool specimens of the AFP case were received in poor
condition.
AFP contact sampling can also be performed either as a part of regular AFP surveillance activities or as
a part of outbreak response activities (Table 5). However, any decision to expand AFP contact
sampling must be made in close consultation between regional and national polio teams and the
laboratory to ensure both that the expansion is justified and that the increase in laboratory workload can
be accommodated.
Table 5. AFP contact sampling during field surveillance and outbreak response
Recommended conditions for AFP contact sampling
Field
surveillance
● All AFP cases with inadequate specimens – i.e., in one or more of the following situations:
- 0 or 1 stool specimen was collected from the AFP case (not 2)
- At least one specimen was collected late, >14 days after onset
- Two specimens were collected less than 24 hours apart
- Specimens arrived in the laboratory in poor condition.
● All AFP cases reported in security-compromised or hard-to-reach areas to expand the
limited opportunity to reach such communities.
Outbreak
response
● All AFP cases reported following confirmation of an outbreak may enhance the sensitivity
of virus detection in an outbreak-affected area.
● AFP contact sampling may be initiated for a limited period in specific geographic areas
(outside the outbreak area and in specific at-risk areas) to enhance the probability of
detecting poliovirus.
AFP = acute flaccid paralysis
AFP contact sampling should not be done when the AFP case has already been confirmed as WPV
or VDPV, as contact sampling will not provide additional information, or when the onset of paralysis of
the AFP case occurred more than 60 days earlier.
5.2 – Conduct AFP contact sampling
AFP contact sampling should be done adhering to a
standardized procedure:
1. Identify potential contacts. Give priority to
younger children (under five years of age) who are in frequent, direct contact with the AFP
case. Include siblings, household members or playmates. If the AFP case stayed in other
locations one week prior to and/or two weeks after paralysis onset, then identify additional
contacts at these locations.
2. Explain the purpose of collecting samples to parents or guardians of the selected contact.
3. Collect one stool sample each from three separate contacts.
4. Follow AFP surveillance protocols for collection, storage, and transport of stool specimens.
5. Fill out a separate laboratory request form for each contact.
6. Each specimen should be labelled clearly as a contact of the AFP case, using the EPID number
of the AFP case with an added contact indicator (“C”) and number; that is, the suffixes: -C1, -
C2, -C3 (Figs. 7 and 8).
Annex guidance
A job aid to support contact sampling is
available in Annex 12. AFP contact sampling.
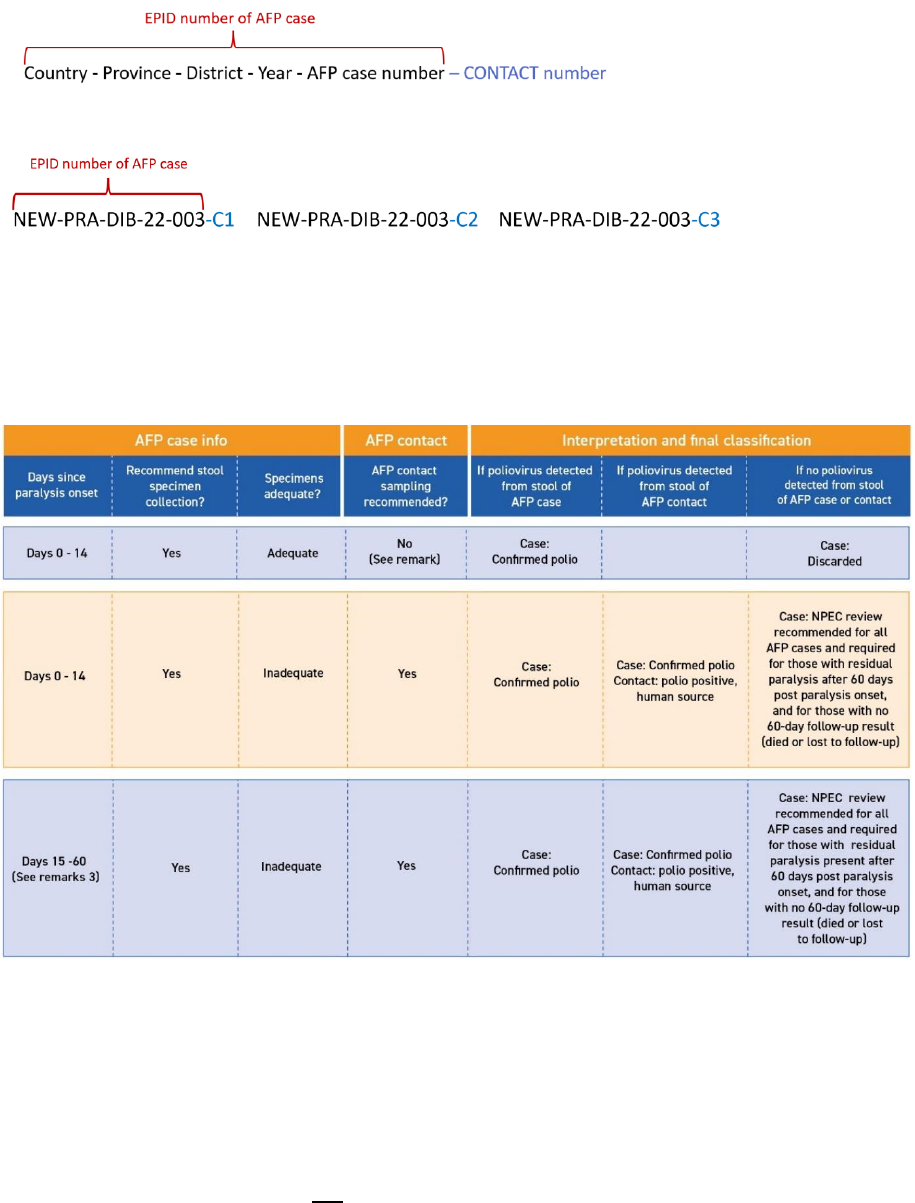
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
26
Fig. 7. Nomenclature for EPID of contacts
Fig. 8. Example of EPIDs for the three contacts of AFP case “NEW-PRA-DIB-22-003”
5.3 – Interpret AFP contact sampling results
Table 6 summarizes how laboratory results of AFP contacts should be interpreted to link AFP cases to
poliovirus epidemiologically.
Table 6. AFP case and contact epidemiological link
AFP = acute flaccid paralysis; NPEC = National Polio Expert Committee
Further details to support interpreting laboratory results on AFP contact sampling:
1. If the AFP case was WPV-negative or VDPV-negative, the isolation of WPV or VDPV from a
contact confirms the AFP case as a WPV or VDPV case, even if the AFP case had adequate
stool specimens.
2. If the AFP case was WPV-positive or VDPV-positive, the isolation of WPV or VDPV from a
contact still represents a programmatically valuable information. However, the virus-positive
contacts of AFP cases are not classified as confirmed poliovirus cases because they do not
meet the case definition, which requires AFP. Results are included as “others” or “other human”
in the poliovirus isolation count.
3. AFP stool specimens collected after 60 days will be considered as inadequate, and no AFP
contact sampling should be conducted. Any positive isolate found in the AFP stool specimen
will be interpreted as an incidental finding, and polio positive human source will not be used as
epidemiological link to confirm poliomyelitis in the AFP case.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
27
6. Laboratory testing and reporting
Sensitive surveillance to detect polioviruses requires effective collaboration between clinicians,
epidemiologists, immunization programmes and laboratories at the national, regional and global levels.
6.1. – The Global Polio Laboratory Network
The Global Polio Laboratory Network (GPLN) was established by the WHO to ensure that high-quality
diagnostic services are available to all countries. The GPLN processes over 220 000 stool samples
from AFP cases and their contacts and more than 12 000 sewage samples per year. As of 2022, the
network consists of 145 WHO-accredited polio laboratories in 92 countries across six WHO regions
(Fig. 9).
Fig. 9. Laboratories within the GPLN by lab role
Source: GPLN, 2021.
WHO-accredited polio laboratories are laboratories that conform to GPLN standards or codes of
practice. The accuracy and quality of testing is monitored by WHO through an annual accreditation
programme that includes onsite reviews of infrastructures, equipment, standard operating procedures
(SOPs), work practices, performance and external proficiency testing. To be included in the network,
laboratories must have the proven capability and capacity to detect, identify and promptly report WPVs
and VDPVs that may be present in clinical and environmental specimens.
The primary roles of GPLN laboratories are to:
• detect poliovirus from stool specimens and sewage samples by isolation using cell culture;
• identify and differentiate isolated polioviruses using intratypic differentiation (ITD);
• genetically characterize poliovirus using sequencing methods, which also determine whether
isolated viruses are wild, vaccine-like or vaccine-derived;
• trace the origin of polioviruses isolated from AFP cases and contacts or from sewage samples;
• maintain a reference bank of nucleotide sequences of known viruses to allow rapid tracing of
the geographic origin of new isolates;
• assess vaccine potency and efficacy if circumstances indicate possible failure;
• conduct serosurveys if knowledge of the antibody status of the population is important; and
• provide evidence that polio has been eradicated.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
28
All national, regional and global polio laboratories in the GPLN follow WHO-recommended procedures
for detecting and characterizing polioviruses from stool specimen and sewage samples derived from
AFP cases/contacts and environmental surveillance, respectively.
6.2 – Coordination between field and laboratory surveillance
Polio field and laboratory surveillance teams work closely to:
• collaborate on surveillance activities that affect workload and testing capacities, such as AFP
contact sampling and targeted healthy children sampling;
• ensure that the laboratory is notified in advance of the shipment of stool specimens;
• ensure that the laboratory provides feedback on the condition of stool specimens, particularly if
there is a need to recollect specimens;
• collaborate on data sharing to ensure accurate case details (e.g., EPID numbers), with corrective
action taken when there are problems;
• share epidemiological findings, laboratory results, classification and genomic sequence results;
• coordinate so there are no discrepancies between the data held by the field team and laboratory to
support the calculation on indicators; and
• reduce the period between the identification of an AFP case and final laboratory results so new
positive cases can be responded to as swiftly as possible. The duration of specimen transport is
used as one of the key indicator for timeliness: ≥80% of stool specimens should arrive at a WHO-
accredited polio laboratory under reverse cold chain conditions within three (3) days of collection
of the second stool specimen collection.
6.3 – Possible laboratory results
Possible laboratory results can include: OPV-like, Sabin-like (SL), or nOPV2-like, WPV, VDPV, non-
polio enteroviruses (NPEV), non-enteroviruses, or no virus isolated (Table 7).
Laboratory tools for polio eradication
Molecular detection and comparative genomic sequencing are major surveillance tools for eradication.
• Poliovirus patterns of transmission can be inferred from analysing patterns of poliovirus evolution.
Poliovirus is a rapidly evolving virus with approximately 1% substitutions per year in the capsid
region of the virus. Viral strain evolution is analysed to estimate the extent and duration of infections
and virus circulation.
• Molecular epidemiological analysis provides additional information to link cases and identify
persistent reservoirs. Sequence comparisons can also determine the source of a poliovirus infection
and distinguish among viruses imported into a new area or country, endemic virus circulation, re-
introduction of poliovirus to a population, and VDPV strains, all of which help to inform eradication
efforts. All WPV and VDPV isolates are subjected to partial (viral protein 1 [VP1] or capsid) or full
genomic sequencing and phylogenetic analysis.
• While interpreting genetic trees, long horizontal branches indicate missing information. Viral
sequences at ends of long branches are called “orphans” if isolates are >1.5% different in the VP1
capsid nucleotide sequence from any isolate previously detected. Isolation of an orphan virus
suggests silent circulation or no detection for an extended period, both of which indicate potential
gaps in surveillance.
At a basic level, results from genomic sequencing help to:
✓ confirm a polio diagnosis;
✓ characterize the poliovirus isolates at the molecular level;
✓ define and monitor how poliovirus is spreading by comparing the nucleotide sequences of different
poliovirus isolates detected over time and in different localities; and
✓ detect specimen or sample cross-contaminations as part of a GPLN quality assurance system.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
29
Orphan virus: The detection of a virus that is >1.5% different in its sequence from any virus previously
detected, referred to as an ‘orphan virus,’ signals the existence of potential gaps in surveillance. As
such, an orphan virus triggers the following actions:
• the immediate (within 24 hours) completion of a detailed case investigation;
• the completion, within 72 hours of receipt of the genetic sequencing result, of an initial risk
assessment to determine the level of risk for further spread and to inform the type and scale of
the response;
• in the event of an outbreak in a previously polio-free country or area: the development of a joint
(MOH and GPEI partners) vaccination and surveillance strengthening response plan and
budget, which may be extended to include several countries, based on the findings of the risk
assessment and depending on the context; and
• the completion of a desk review and a field investigation aimed at identifying possible
surveillance gaps and missed transmission. Identifying the population group(s) or geographic
area(s) the virus may have been circulating in undetected is especially important, as it enables
the programme to hone its response by developing surveillance interventions tailored for these
specific population group(s) or area(s).
Table 7. Possible laboratory results from the testing of stool and environmental samples
Lab results
Type of virus
Reported as
OPV-like or Sabin-like (SL),
or nOPV2-like
Vaccine strain poliovirus type 1, 2 or 3
SL1, SL2, SL3, nOPV-like
Wild poliovirus
Wild poliovirus type 1, 2 or 3
WPV1, WPV2, and WPV3
Vaccine-derived poliovirus
Vaccine-derived poliovirus type 1, 2 or 3, further
classified as:
● circulating VDPVs (cVDPVs)
● immunodeficiency-associated VDPVs
(iVDPVs)
● ambiguous VDPV (aVDPV)
This is done by combining laboratory results with
epidemiological and clinical information.
* For nOPV2, specific terminology will be used
when sufficient data will be gathered
VDPV1, VDPV2, VDPV3,
further reported as:
● cVDPV1, cVDPV2, cVDPV3
● iVDPV1, iVDPV2, iVDPV3
● aVDPV1, aVDPV2, aVDPV3
Non-polio enteroviruses
Non-polio enteroviruses
NPEV or NPENT
Non-enteroviruses
Non enteroviruses
NEV
No virus isolated
No virus isolated
NVI
aVDPV = ambiguous vaccine-derived poliovirus; cVDPV = circulating vaccine-derived poliovirus (types 1,2,3); iVDPV = immunodeficiency-
associated vaccine derived poliovirus (types 1,2,3); NEV = non-enterovirus; nOPV = novel oral polio vaccine; nOPV2 = novel oral polio
vaccine type 2; NPENT = non-polio enterovirus; NPEV = non-polio enterovirus; NVI = no virus isolated; OPV = oral polio vaccine; SL =
Sabin-like (types 1,2,3); VDPV = vaccine-derived poliovirus (types 1,2,3); WPV = wild poliovirus (types 1,2,3)
A combination of findings is possible for: OPV-like, SL, nOPV-like; WPV; VDPV; and NPEV. Results
that fall under the second or third categories (i.e., WPV or VDPV) may indicate an event or outbreak
and should be followed by appropriate response. All results should be communicated to all relevant
administrative levels of the polio eradication programme, as well as the submitting physician or health
facility. If available, further clinical management can be offered by the attending physician, or a polio
rehabilitation programme in some countries.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
30
6.4 – Monitoring laboratory timeliness
The GPLN routinely measures the timeliness of the work done in its laboratories, with the following
indicators for stool specimen processing and their targets (see also Annex 3. Indicators for AFP
Surveillance).
• ≥80% of specimens with final results available within 21 days of receipt from a direct detection
country OR within 28 days of receipt from a non-direct detection country at a WHO-accredited
polio laboratory.
• ≥80% of specimens with WPV/VDPV final results available within 21 days of receipt from a
direct detection country OR within 28 days of receipt from a non-direct detection country at a
WHO-accredited polio laboratory.
• ≥80% of poliovirus specimens with sequencing results available within 7 days of receipt of
isolate at a WHO-accredited Polio sequencing laboratory.
The overall target and indicator for the timeliness of obtaining final laboratory results (interval from
paralysis onset to specimen testing and result) is:
• ≥80% of WPVs and VDPVs reporting final laboratory results within 35 days of AFP onset.
7. 60-day follow-up investigation
7.1 - Determine which cases should undergo a 60-day follow-up examination
The hallmark of poliomyelitis is that most paralytic cases will not fully recover but will suffer permanent
residual neurological sequelae, or residual paralysis. All surviving AFP cases should therefore be
examined again for residual paralysis between the 60
th
and the 90
th
day after the onset of paralysis. The
presence of residual paralysis at that time could be further evidence that the cause of paralysis was due
to the poliovirus.
The 60-day follow-up examination is especially
important for AFP cases with no stool specimen
collected or inadequate specimens, for which
reliable laboratory results may not be available.
The presence of residual paralysis upon follow-
up will be a key element for the NPEC to
consider in their final case classification. The
programme therefore strongly recommends that
all AFP cases with inadequate specimens
receive a 60-day follow-up examination.
Likewise, given the programmatic importance of
vaccine viruses (e.g., Sabin, Sabin-like viruses, nOPV2, nOPV2-like viruses), the programme strongly
recommends that all AFP cases with vaccine-type (Sabin-type, nOPV2 type) poliovirus in their
stool specimens receive a 60-day follow-up examination. This facilitates a later possible diagnosis of
vaccine-associated paralytic polio (VAPP).
In some WHO regions, such as the Region of the Americas and the Eastern Mediterranean Region, a
60-day follow-up examination is required for all AFP cases, irrespective of stool specimen’s condition,
as the exam provides valuable information to allocate a final diagnosis to those non-polio AFP cases.
7.2 – Conduct a 60-day follow-up examination
The result of the 60-day follow-up examination depends considerably on the experience and clinical
skills of the person conducting the exam. This examination should ideally be conducted by a
paediatrician experienced in examining children. Well-trained paediatricians will detect even small
degrees of residual weakness which less trained health workers may not be able to find. It is also
preferred to have it done by the physician/officer who initially examined the case.
When is a follow-up exam required?
Ideally, all AFP cases should undergo a 60-day
follow-up examination. However, a follow-up exam
is required for the following:
• AFP cases without stool specimen collection or
for which only inadequate stool specimens
could be collected; and
• AFP cases with isolation of vaccine-type
(Sabin-type, nOPV-type) poliovirus.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
31
A 60-day follow-up examination is conducted using both the original CIF and the 60-day follow-up
examination form (Annex 7. Examples of forms). During the exam, the clinician or officer should
systematically assess the patient.
60-day follow-up examination process
1. Verify with the family that all information on the previously documented CIF is correct.
2. Inquire if the paralysis or weakness has improved, has remained the same, or has progressed.
3. Observe how the child moves their limbs or affected areas of the body. Watch the child walk, or
move arms, and look for signs of atrophy.
4. Examine muscle tone, power, and reflexes. Verify sensation.
5. Even mild residual weakness should be considered as ‘residual paralysis.’
6. Complete the 60-day follow-up examination form and send it to the national Expanded
Programme on Immunization (EPI) or polio programme.
8. Final AFP case classification
Once laboratory results have been received, all AFP cases undergo final case classification. The target
is to classify all cases within 90 days of the onset of paralysis.
The final classification of cases with inadequate stools is done by the National Polio Expert Committee
(NPEC). Depending on the region, this committee may also be known as National Polio Expert Group,
National Polio Expert Review Committee (with the acronyms ERC or NEC) or National Polio Expert
Panel. (See Annex 15. Polio committees and commissions.)
National Polio Expert Committee (NPEC)
The NPEC is an honorary, volunteer group of paediatricians, neurologists, virologists and
epidemiologists that meets regularly and on an ad hoc basis, generally between once a month to four
times a year. The committee’s membership varies in size and composition. Its role is to:
• classify all AFP cases but, at a minimum, all AFP cases with inadequate stool specimens that
have residual paralysis at 60-day follow-up, that have died or are lost to follow-up;
• review cases with suspected VAPP, which is assigned after excluding all possible diagnoses.
(VAPP cases with a history of receiving nOPV2 should be referred to the causality assessment
committee to assess an association with the use of nOPV2. In some countries, the NPEC
performs causality assessment as part of its terms of reference. See Annex 16. Safety
surveillance for nOPV2.);
• provide technical advice pertaining to AFP cases and final diagnosis (if appropriate); and
• monitor quality of the AFP surveillance system in general.
8.1 – Determine final AFP case classification
In reviewing all AFP cases, The NPEC provides final case classification (Fig. 10).
AFP cases with adequate specimens are either:
• confirmed as polio, if WPV or VDPV was detected in any stool specimens from either the case
or contacts; or
• discarded as non-polio AFP, if no WPV or VDPV was detected in adequate stool specimens
from either the case or contacts.
AFP with inadequate specimens will be:
• confirmed as polio if WPV or VDPV was detected in any stool specimens from either the case
or contacts;
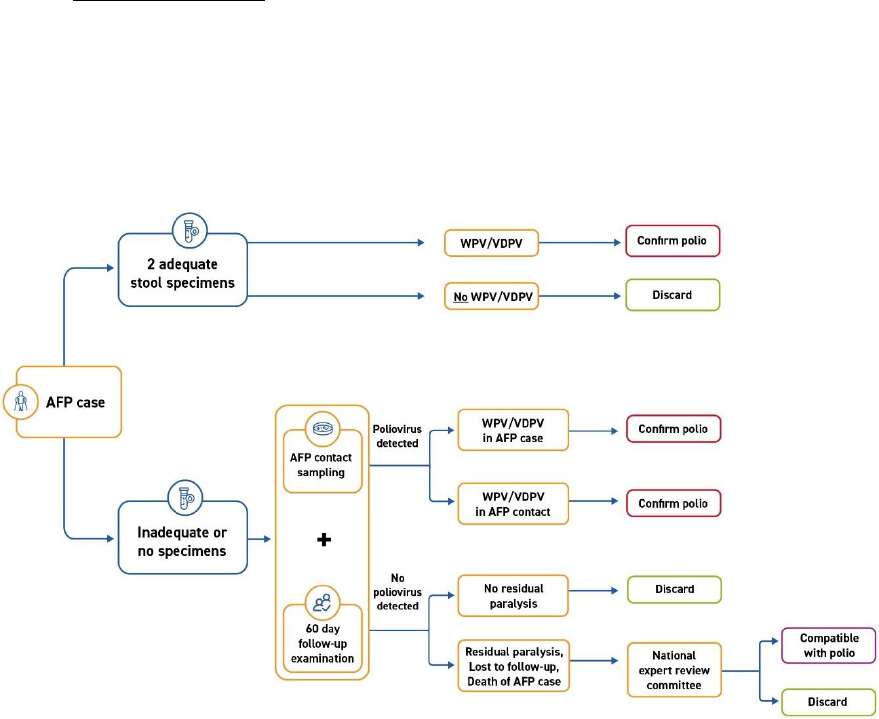
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
32
• compatible if the NPEC has concluded so after reviewing that (1) no WPV or VDPV was
detected in any stool specimen from either the case or its contacts, and that (2) there is residual
paralysis (or weakness) at the time of the 60-day follow-up visit, or that the follow-up was not
done due to death or loss to follow-up of the case, and (3) upon review, the possibility of polio
could not be ruled out; or
• discarded as non-polio AFP, if no poliovirus was detected from the case or his/her contacts,
and no residual paralysis was observed at the 60-day follow-up visit of the case, or if the NPEC
concludes after reviewing that (1) no poliovirus was detected in any stool specimens from either
the case or contacts, and that (2) even though there was residual paralysis, or the case was
lost to follow-up, or had died, there was sufficient evidence (clinical evidence and supportive
documentation) to discard the case as non-polio.
Fig. 10. Virologic AFP classification scheme
AFP = acute flaccid paralysis; VDPV = vaccine-derived poliovirus; WPV = wild poliovirus
Source: WHO.
Non-polio and polio-compatible cases
For cases classified as non-polio AFP and for which no prior working diagnosis was given, the NPEC
will be expected to assign a final diagnosis based on all information at its disposal, such as the initial
investigation, the 60-day follow-up examination results, and other clinical evidence.
Polio-compatible cases can only be classified as such by the NPEC. Those cases are neither confirmed
as polio nor discarded as non-polio. Such cases are important because they indicate a surveillance
failure in any of the steps required to collect adequate specimens, from delays in the AFP case seeking
health care to specimens received at a WHO-accredited polio laboratory in good condition. A cluster of
polio compatible cases in a short period of time is worrisome as the programme cannot rule out polio as
one of the reasons for this cluster of AFP cases.
Regular mapping and review of polio-compatible cases helps to find areas with poor surveillance to
address the underlying problem that has caused late specimen collection.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
33
8.2 – Further investigate, if needed
Certain critical situations require further investigation to supplement the initial case investigation and
gain a better understanding of the context and circumstance of the case or cluster of cases and thus
uncover possible reasons for the occurrence and assess the risk of virus spread if present.
Any one of the following situations warrants a prompt detailed case investigation:
• a single isolate of WPV through AFP or ES;
• a single isolate of VDPV1, VDPV2 or VDPV3 through AFP or ES;
• any SL2 poliovirus in an area with no recent vaccination campaign with type 2-containing
vaccine;
• a clustering of AFP cases classified as polio-compatibles, i.e., usually defined as two or more
cases in either a single district or two neighbouring districts within four weeks;
• a clustering of AFP cases within a district or in neighbouring districts, i.e., at least twice the
number of expected AFP cases reported within a month, in a limited geographical area (please
refer to Table 11b in Monitoring); and
• in some cases, a “hot” AFP case in advance of laboratory confirmation.
15
The main elements to launch a detailed case investigation are included in the Detailed Case
Investigation Form or Report (Annex 7. Examples of Forms).
16
The form compiles information on the
case (or environmental site), as well as information about the community (or catchment area).
The objectives of detailed investigations are to:
• define characteristics of the case(s), including demographics and socio-cultural aspects to
better identify and address possible risk factors;
• identify possible origins or causes for the virus circulation or source of importation of poliovirus;
• assess the potential spread of the virus by looking for some unreported cases in the area; and
• formulate control measures (immunization and surveillance) to interrupt the transmission and
prevent spread or improve the ability to detect circulation.
Following the detailed case investigation of any polio event or outbreak, it is critical to assess and
enhance poliovirus surveillance.
17
15
A “hot” AFP case is a case that looks clinically like polio (rapid progression of paralysis; asymmetrical paralysis; fever at onset)
plus or minus any of the following criteria as defined by the country or region: less than five years of age; fewer than three doses
of polio vaccine or unknown vaccination status, contact with infected area. See Table 11a in Monitoring for further information on
“hot” cases.
16
An example of a detailed case investigation form can be found on the GPEI website (http://polioeradication.org/wp-
content/uploads/2016/09/Detailed-Case-Investigation-Form_July2011_EN.doc).
17
Global Polio Eradication Initiative (GPEI). Standard operating procedures: responding to a poliovirus event or outbreak, version
4. Geneva: World Health Organization; 2022 (https://polioeradication.org/wp-content/uploads/2022/07/Standard-Operating-
Procedures-For-Responding-to-a-Poliovirus-Event-Or-Outbreak-20220807-EN-Final.pdf). Global Polio Eradication Initiative
(GPEI). Interim Quick Reference on Strengthening Polio Surveillance during a Poliovirus Outbreak. Geneva: World Health
Organization; undated (https://polioeradication.org/wp-content/uploads/2021/12/Quick-Reference_Strengthening-Surveillance-
during-Poliovirus-Outbreaks_24-March-2021.pdf).
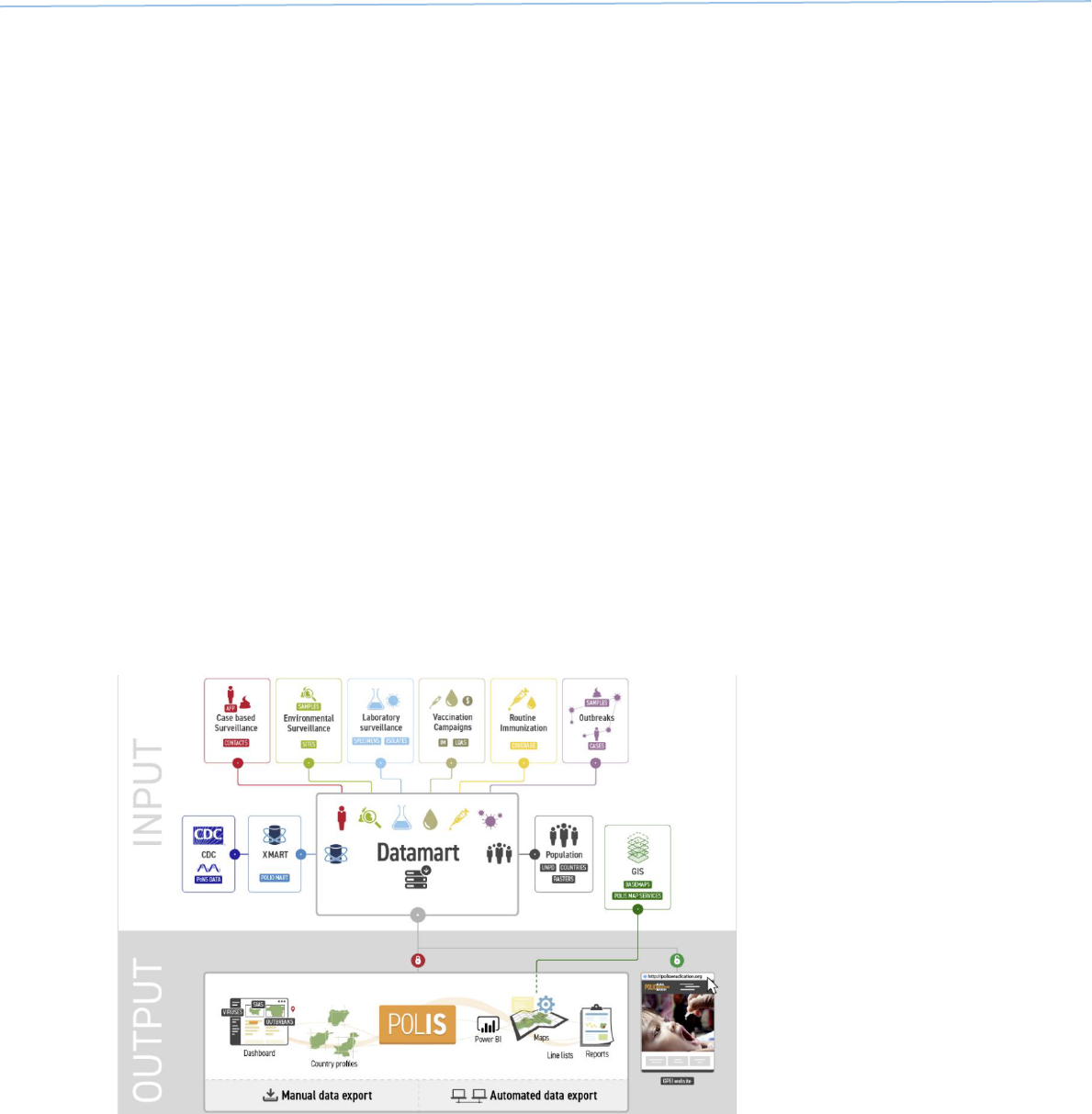
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
34
MONITORING AFP surveillance
1. Data management
Data that are complete, accurate and timely are key to monitoring progress toward eradication. For data
to be of use, data collection and processing tools must be used correctly, and the data must be
analysed on a regular basis and interpreted properly to produce information to support decision making.
The programme gathers data from several sources for acute flaccid paralysis (AFP) surveillance:
• Case-based AFP data is collected through case investigation forms (CIFs) and 60-day follow-
up exams, where it is compiled in a database and shared weekly with WHO regional offices and
headquarters. It is also placed on an online platform, the Polio Information System (POLIS).
• Specimen-based data on AFP cases, case contacts and targeted healthy children stool
specimens is gathered from the laboratory, compiled in databases and shared weekly with both
WHO regional offices and headquarters. It is also placed on POLIS.
• Genetic sequencing results for poliovirus isolates also provide a source of data for AFP
surveillance, some of which are placed on POLIS.
• Routine surveillance data (“zero-reporting”) is collected from all reporting sites and compiled at
national level
• Active surveillance data is collected from AS visits conducted by surveillance officers and
should be compiled at national level.
1.1 – Polio Information System (POLIS)
Hosted at the WHO headquarters, POLIS consolidates, harmonizes, performs quality checks and
analyses data from AFP surveillance, environmental surveillance (ES), supplemental immunization
activities (SIAs), and laboratory testing.
18
POLIS thus offers a central repository that permits access to
standardized data, reports and outputs by country programmes and partners (Fig. 11).
Fig. 11. POLIS visualization
18
POLIS can be accessed online at: https://extranet.who.int/polis/Help (log-in required).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
35
Broadly, AFP surveillance data management is indispensable to support decision-making (Table 8).
With a focus on AFP surveillance, the role of data managers is to ensure that:
• AFP data is collected and shared, where applicable, in a timely manner;
• AFP data is complete and free of data entry errors (data quality checks);
• AFP data is accurate (e.g., logical chronology of dates); and
• AFP data is filed and archived properly.
In addition, and together with surveillance officers, data managers ensure that:
• accurate and up-to-date data is analysed, and information is presented clearly so as to best
support data-driven decision making; and
• reports and feedback are complete and provided in a timely manner, particularly as they
support monitoring surveillance performance.
Table 8. The uses of AFP surveillance data to programme decision-makers
Country context
Use of AFP surveillance data
All countries
● Calculate standard AFP quality indicators for surveillance performance
● Focus corrective efforts on low-performing areas
All countries
● Provide evidence on surveillance quality to national and regional certification
bodies as the basis for regional and global polio-free certification
Endemic countries,
outbreak areas
● Track WPV, VDPV circulation to inform immunization activities and monitor
progress towards interrupting transmission
AFP = acute flaccid paralysis; VDPV = vaccine-derived poliovirus; WPV = wild poliovirus
5.2 – Mobile applications and mobile data collection
New technologies can help improve surveillance processes and data management. Such innovations
have traditionally been used to improve timeliness in the collection, storage and dissemination of data
and to improve monitoring and supervision activities. Innovation can also be used to locate populations
and get a better understanding of the scope of the surveillance network.
The widespread use of mobile devices facilitates cleaner, faster and more reliable data capture and
increases communication between surveillance officers and the healthcare network. Many successful
innovations with mobile devices are currently in use across the polio programme (Table 9). It is
recommended that country programmes consult with WHO regional offices to make sure certain data
standards are met and ensure data can be captured in POLIS.
Table 9. Examples of successful polio programme innovations
Innovation
Definition
Benefits
Tool
E-surv
Electronic
surveillance
Real-time monitoring and
reporting system on active
surveillance (AS) visits.
● Registers time, location and record
data on AS visits.
● Tracks the coverage of AS visits
Mobile phone or
tablet
ISS
Integrated
supportive
supervision
Real-time monitoring and
reporting system on
supervisory visits for
essential immunization, cold
chain and vaccines, and
incidence of VPDs.
● Registers time, location and record
data on supervisory visits
● Tracks coverage of supervisory
visits
● Displays trends across time and
geographies
Mobile phone or
tablet
AS = active surveillance; E-surv = electronic surveillance; ISS = integrated supportive supervision; VPD = vaccine-preventable disease
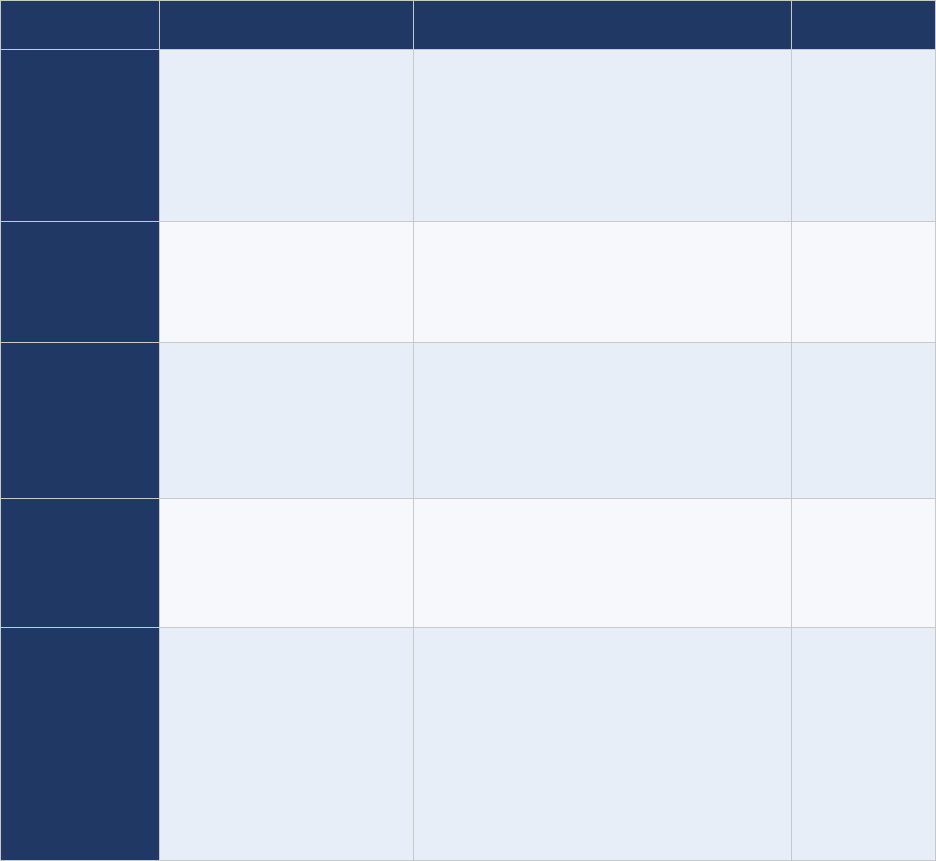
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
36
Table 9 (continued)
Innovation
Definition
Benefits
Tool
AVADAR
Auto-Visual AFP
Detection and
Reporting*
Reporting and monitoring
tool for CBS to enable
community members (i.e.,
birth attendants, traditional
healers, village healers) to
detect and report AFP cases
● Reminder to look for AFP cases
● Time and location of notification of
“suspected AFP case”
● Directs electronic notification of
suspect AFP case to supervisor(s)
Mobile phone
or tablet
Geo-
localization
Mobile devices with global
positioning system (GPS)
receivers can allow
geolocation of cases
● Allows exact localization of AFP cases
or health facilities
Mobile phone
or tablet
WebIFA
Web Information
For Action
Designed to collect, report
and analyse surveillance
data using a mobile device
● Centralized and harmonized data from
field collection and laboratory reporting
for AFP, environmental, and iVDPV
surveillance
● Improves data quality, streamlines
workflow between surveillance teams
Mobile phone
or tablet,
computer
Barcode
QR code system to track
samples from collection to
testing
● Real-time tracking of samples
● Avoids data entry errors
● Linked to WebIFA for tracking and data
verification
Mobile phone
or tablets
Currently being
pilot tested
WhatsApp
Chat groups
● Improves communication within
surveillance teams, strengthens and
connects teams
● Supports direct information
dissemination and issue resolution.
● Motivates frontline surveillance efforts,
provides training opportunities by
taking and sharing pictures of their
work.
Mobile phone
*Information on AVADAR is available online (https://www.ehealthafrica.org/avadar), as well as in Diallo M, Traore A, Nzioki MM et al. Auto
Visual AFP Detection and Response (AVADAR) Improved Polio Surveillance in Lake Chad Polio Outbreak Priority Districts. J.
Immunological. Sci. (2021); S (002): 73-85 (https://doi.org/10.29245/2578-3009/2021/S2.1101).
AFP = acute flaccid paralysis; CBS = community-based surveillance; GPS = global positioning system; iVDPV = immunodeficiency-
associated vaccine-derived poliovirus; QR code = quick response code; WebIFA = web-based information-for-action system
5.3 – Geographic information system (GIS) mapping
GIS mapping and satellite imagery are also useful to identify and locate populations and catchment
areas. GIS is now widely used by the programme for vaccination campaigns but also in the context of
surveillance to:
• map surveillance network and AFP cases to ensure that populations are covered by the
surveillance network; and
• better understand population movements and where populations are located. This helps to
understand the performance of the surveillance system (indicators) and areas where
surveillance strategies need to be adapted (e.g., hard-to-reach populations).
While not possible in all contexts, the wider deployment and use of GIS mapping and satellite imagery
is encouraged, including to capture the GPS coordinates of where AFP cases reside, of health facilities,
reporting sites, etc., and to better visualize catchment areas.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
37
2. Monitoring
Monitoring should be conducted on a regular basis and should highlight both trends and anomalies in
the performance and quality of surveillance.
2.1 – Collect, analyse, and use data
Data should be consolidated and analysed at district, provincial and national levels to assess the
sensitivity, timeliness and quality of surveillance. All data should be updated promptly in the event of an
error. Data should also be updated after laboratory results are received and once a final case
classification is assigned.
Monitoring should be done:
• for case- and specimen-level data (line listing) monitor the quality of case investigations
(including completeness of forms) and ensure accurate and up-to-date case- and specimen-
based data is available for performance analyses;
• for site visits, including active surveillance (AS) and supervisory monitor completeness and
timeliness of AS and supervisory visits and related data; and
• for reports, including AS and zero-reporting monitor completeness of data and timeliness of
report.
Data should be disaggregated by space and time:
• within and/or across geographies: local, district, province, national; and
• over time: by month, by quarter, semester, yearly.
Data should also be stratified, where possible and whenever a more descriptive analysis is required:
• by sex (e.g., “number of unreported AFP cases by sex identified during AS visits”);
• by special population group (e.g., “number of AFP cases reported by category of special
population”); and
• by health-seeking behaviour (e.g., “number of AFP cases with ≤2 health encounters between
onset and notification / number of AFP cases (stratify by sex)”).
Routine analyses include the following set of reports and products:
• graph of confirmed polio cases by year (indicates progress made towards eradicating polio);
• graph of confirmed polio cases by month (indicates the season of high and low polio;
transmission and is useful for planning supplementary immunization activities [SIAs]);
• dot map of confirmed polio cases (shows where poliovirus is circulating and high-risk areas to
be targeted with special strategies);
• dot map of AFP cases and compatible cases (identifies possible areas of low performance);
• table showing the key indicators by the first administrative level (see Annex 3);
• disaggregation of indicators by sex and by special population/high-risk groups or areas (helps
pinpoint possible reasons for suboptimal performance or gaps in surveillance; hence can direct
to possible solutions); and
• graph of OPV/IPV status of non-polio AFP cases aged 6-59 months (indicates whether
immunization efforts should be intensified and areas of possible risk of virus emergence and/or
spread).
AFP surveillance indicators
Performance indicators are used to monitor the quality of disease surveillance and laboratory
performance using both core and non-core indicators. For a comprehensive list, see Annex 3.
Indicators for AFP surveillance.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
38
Two indicators remain the gold standard to assess AFP surveillance quality:
✓ non-polio AFP rate, and
✓ stool adequacy.
Indicators for the timeliness of activities, as introduced in the GPEI 2022-2026 Strategy, are of particular
importance (Table 10). They generally only apply to outbreak and at-risk countries. Delays in detection
can happen at any stage of field, logistic, and laboratory activities. Countries must monitor timeliness at
every stage of the process. Annex 14 provides insight into causes of delays and ways the programme
can address them.
Table 10. AFP surveillance indicators related to timeliness
Timeliness of
Indicator
Detection
# of AFP cases with WPV/VDPV final laboratory results ≤ 35 days of onset
Notification
# of AFP cases reported within 7 days of paralysis onset
Investigation
# of AFP cases investigated within 48 hours of notification
Stool collection
# of AFP cases with 2 samples collected ≥ 24 hours, both within 11 days of paralysis onset
AFP = acute flaccid paralysis; VDPV = vaccine-derived poliovirus; WPV = wild poliovirus
2.2 – Report on progress and provide feedback
Progress reports: Weekly, monthly and/or quarterly
reports on AFP surveillance sensitivity and quality are
critical to maintaining effective surveillance and keeping
health staff and concerned parties (both local and
international) engaged.
Similarly, periodical progress reports to local, regional,
and global actors, as well as the media, are needed to
maintain awareness of polio and a commitment to the wider goal of eradication.
Feedback: Providing written feedback within a week of receiving reports and conducting supervisory
visits is crucial to address identified gaps in surveillance, some of which can be due to insufficient
training or dwindling motivation. If no issues are noted, supervisors should provide feedback in the form
of acknowledging receipt of the report with thanks.
Furthermore, providing feedback information to all designated reporting sites is needed to:
• report progress and problems;
• compare performance across the country;
• facilitate discussions on inaccuracies in data, surveillance gaps, and ways to close gaps;
• encourage complete, timely reporting and inform concerned parties of programme progress;
and
• motivate health staff/agents.
Responding to AFP surveillance data (using data for action)
Both data managers and surveillance officers should monitor and analyse AFP data routinely and go
beyond the regular indicators to identify issues that may point to gaps in surveillance and allow the early
detection of outbreaks. Issues may include anomalies, such as a sudden drop in performance or an
increase in the number of AFP cases reported, or unusual trends or patterns, such as repeated,
periodical drops in the timeliness of reporting (Tables 11a and 11b). Annex 3 lists both core and non-
core indicators, which provide an additional means of looking at available data beyond the regular
indicators.
A monthly polio surveillance report
(or a polio update in an integrated
surveillance report) should be produced at
the national level and shared with the
entire surveillance network, including
programme partners and reporting sites.
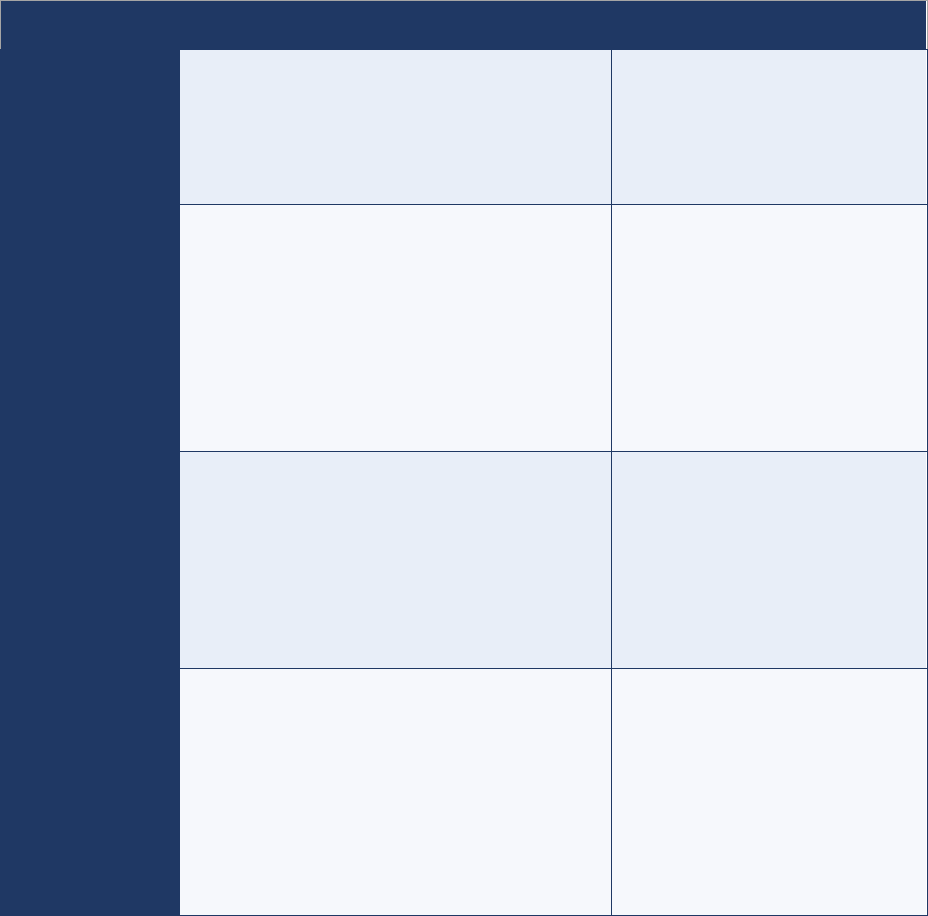
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
39
Analysing beyond the indicators is done by:
• reviewing line listings for AFP, AFP contact and healthy children, and laboratories;
• reviewing CIFs retrospectively over a determined period (generally three or six months); and
• disaggregating data by sex and special population/high-risk groups, as well as performing
health-seeking behaviour analyses. (See Annex 9. Health-seeking behaviour)
Table 11a. Virus and performance triggers for responding to AFP surveillance data
Situation
Description
What to do
Underperforming
areas
Areas that record low performance in key indicators such
as non-polio AFP rates or stool adequacy (or, on the
contrary, experience a sudden increase in the number of
AFP cases reported); areas whose performance
intermittently falls below expectations such as repeated
drops in timeliness of reporting.
● Follow-up by visits, telephone,
e-mail to identify reasons for the
performance issue.
● Address any problems immediately
(e.g., re-training, lack of
resources…)
Silent areas
The definition of “silent” is country-specific and usually
refers to any area (province or district) that should have
but didn’t report at least one AFP case (based on time
and under 15 population). That is, an area (usually a
district) that did not report a single AFP case in a period
varying from 6–12 months or more depending on the
population size and expected AFP case reporting, taking
into consideration that the non-polio AFP rate is1/100 000
or more depending on the polio eradication situation
(certified polio-free, endemic, outbreak).
● Issue an alert or other
communication to district team that
highlights the potential gap
● Review the surveillance functioning
and process (including AS) and
conduct sensitization activities
● Conduct full surveillance review
(if required)
● Trigger an ad hoc case search in
heath facilities
Data “too good to
be true”
Indicators that show unusually and unexpectedly high
performance, e.g., close to 100% of AFP cases have 2
stools collected ≤14 days post paralysis onset. Possible
reasons include: cases detected more than 14 days after
onset are not being reported; or, the reporting date is
being changed to <=14 days after onset.
● Check for manual errors or issues
with data manipulation or migration.
● Seek confirmation with the data
manager (and surveillance officer,
if needed) who collected and
entered the data
● Review CIFs and proceed to field
validation of cases/questionable
CIFs, if needed.
“Hot” cases
AFP cases that clinically looks like polio by meeting all
three cardinal signs of poliomyelitis: rapid progression of
paralysis; asymmetrical paralysis; and fever at onset.
Additional criteria, as defined by the country or region
depending on epidemiology, may include: less than five
years of age; fewer than three doses of polio containing
vaccine or have an unknown vaccination status; contact
with areas/groups with recent virus circulation. The
identification of a “hot case” must trigger the fast-tracking
of specimen processing by the laboratory.
● Ensure the stool specimens reach
the laboratory as quickly as
possible and priority is given for lab
processing.
● Prioritize field investigation
● Check for possible clustering of
(other) “hot cases.” In the event of
a cluster, follow instructions for
clustering (see below).
AFP = acute flaccid paralysis; AS = active surveillance; CIF = case investigation form
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
40
Table 11a (continued)
Situation
Description
What to do
Over-discarding
cases and
“potential
compatible”
cases
AFP cases that may be considered as “potentially polio
compatible” have inadequate stools specimens and either
a) have a 60-day follow-up finding as: residual paralysis
or “lost to follow-up” or “died before follow-up” or b) have
not received any 60-day follow-up visit and have not been
classified or have been “discarded” by the NPEC. The
existence of such cases may flag an “over-discarding” of
cases by the NPEC, which rejected these cases as “non-
polio” when there was potentially a justification to classify
them as “polio compatible.” A clustering in time and space
of such cases of concern (i.e., cases with inadequate
specimens, residual paralysis that were discarded),
should be investigated promptly.
● Check for possible clustering of
(other) “potentially compatible”
cases (using the AFP line list). In
the event of a cluster, follow
instructions for clustering (see
below).
● Consider having the NPEC
members re-oriented.
“Breakthrough”
transmission
Any WPV or cVDPV detected in an AFP case, healthy
child or ES with the date of onset of paralysis (for AFP
cases) or the date of sample collection (for healthy child
or ES) >21 days after the first day of the last SIA in an
area where at least two SIAs have been implemented is
evidence of breakthrough transmission. Where there is a
high-risk of continued circulation, a shorter threshold of
14 days rather than 21 may be considered, e.g.,
inaccessibility, evidence of very poor quality SIAs,
surveillance gaps.
● Conduct thorough field
investigation and risk assessment
(epidemiology, surveillance quality
and sensitivity, as well as
campaign quality). Any decision on
additional campaigns will depend
on the result of these activities.
AFP = acute flaccid paralysis; cVDPV = circulating vaccine-derived poliovirus; ES = environmental surveillance; NPEC = National Polio
Expert Committee; SIA = supplementary immunization activity; WPV = wild poliovirus
Table 11b. Cluster-specific triggers for responding to AFP surveillance data
Description of clusters
What to do
The detection of at least two times
the number of expected AFP
cases occurring in a district (or
province in small countries) within
a month period.
Look out for clusters of
polio-compatible cases,
“hot” cases, “potential compatible”
cases, or “zero-dose” cases.
Possible reasons for clusters:
● More than one polio case
has occurred due to a
new importation or
emergence of poliovirus.
● A relative increase in the
number of non-polio AFP
cases detected and
reported in area due to an
increased sensitization or
search for AFP.
Cluster investigations are similar to polio outbreak investigations. It includes:
● Detailed case investigation: validating information, dates, doses, more info on
movement, visitors, links with other cases
● Looking for more cases and viruses / surveillance assessment and
enhancement
● Active case search in community and health facilities.
● Raise awareness through meeting and interpersonal communication.
● Assess surveillance performance and identify possible gaps.
● ensure that all the high-risk groups are covered by surveillance and that their
health-seeking behaviour is taken into consideration.
● Assessing the risk for virus emergence or importation as well as possible
spread and its direction: review of immunization activities and coverage which
is in favour of possible VDPV emergence/WPV1 importation, investigating the
sociocultural characteristics of the area, pop density and population movement
in and out of the area.
● It is important to flag specimens of these cases and their contacts in the lab for
fast tracking and prioritization and continue sensitization and enhancement of
surveillance activities in the district and connected areas.
AFP = acute flaccid paralysis; VDPV = vaccine-derived poliovirus; WPV1 = wild poliovirus type 1

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
41
3. Evaluation
Evaluations can take the form of audits and desk or field reviews. For outbreak-affected countries,
outbreak response assessments (OBRAs) are also conducted.
3.1 – Conduct audits
All countries benefit from internal annual audits of their AFP surveillance system to assess, identify and
respond to subnational performance gaps. The findings of an audit are particularly useful for annual
surveillance planning.
Audits involve carrying out analyses on data that has been disaggregated by high-risk status, sex and
health-seeking behaviour. They also explore context-specific risk factors, such as special populations or
hard-to-reach geographies. Audits should include all components of the AFP surveillance system:
passive reporting, AS visits and coverage, CBS, staffing and more. Audits are typically performed
internally by the national team and may include desk and/or field assessments.
3.2 – Conduct desk and field surveillance reviews
Periodic evaluations of AFP surveillance systems are done through desk reviews, often followed by field
reviews.
• Desk reviews thoroughly review existing data and analyse indicators to assess overall AFP
surveillance performance. Desk reviews provide an overview of surveillance sensitivity over a
defined period, usually three years, and aim to highlight possible gaps. These reviews can be
done at the office, i.e., at a “desk,” unlike field reviews that involve site visits.
• Field reviews build on desk reviews by targeting a set of provinces or districts for visits. Field
reviews are conducted by a team of peer (internal) reviewers or a mix of internal and external
reviewers, if the field review has international participation, to assess the performance of the
surveillance system and the quality of the surveillance network.
Recommendations from desk and field reviews are translated into a surveillance plan to either maintain
the level achieved or to strengthen where gaps were identified. Depending on the purpose and scope of
these reviews, special attention may be paid to high-risk, access-compromised and hard-to-reach areas
and populations as these areas and populations require special strategies and added resources, which
should be the object of periodical assessments.
3.3 – Conduct outbreak response assessments (OBRAs)
Poliovirus surveillance quality is a key component of
outbreak response assessments (OBRAs), conducted
by the GPEI for all polio outbreaks. OBRAs assess
whether vaccination and surveillance activities are
robust enough to detect and stop poliovirus
transmission. They also identify further activities to
address remaining gaps and interrupt transmission of
the outbreak virus.
OBRAs are conducted regularly throughout an outbreak until an OBRA mission declares the outbreak
to be over. Closure of the outbreak can only be done if there is evidence of high-quality surveillance
sensitivity.
19
19
For OBRA resources, see: Global Polio Eradication Initiative (GPEI). Aide-mémoire on OBRAs, version 2. Geneva: World
Health Oragnization; 2019 (http://polioeradication.org/wp-content/uploads/2016/07/Polio-Outbreak-Response-Assessment-
English-Version-2-December-2019-201912.pdf). Global Polio Eradication Initiative (GPEI). Standard operating procedures:
responding to a poliovirus event or outbreak, version 4. Geneva: World Health Organization; 2022 (https://polioeradication.org/wp-
content/uploads/2022/07/Standard-Operating-Procedures-For-Responding-to-a-Poliovirus-Event-Or-Outbreak-20220807-EN-
Final.pdf). Global Polio Eradication Initiative (GPEI). Interim Quick Reference on Strengthening Polio Surveillance during a
Poliovirus Outbreak. Geneva: World Health Organization; undated (https://polioeradication.org/wp-
content/uploads/2021/12/Quick-Reference_Strengthening-Surveillance-during-Poliovirus-Outbreaks_24-March-2021.pdf).
For outbreak-affected countries that use
nOPV2 as part of their response, the GPEI
has provided nOPV2 specific guidance.
Download Polio Field and Laboratory
Surveillance Requirements in the
Context of nOPV2 Use

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
42
SUSTAINING AFP surveillance
1. Building a skilled workforce
To ensure that all acute flaccid paralysis (AFP) surveillance stakeholders have up-to-date technical and
interpersonal skills, human resources administrators should work together with surveillance supervisors
and managers to select, train, support and retain an effective and motivated surveillance workforce.
1. Selection: The selection of surveillance officers, supervisors, routine surveillance focal points and
community-based surveillance (CBS) informants should be based on a candidate’s ability to perform the
role and their potential for development. Gender balance and appropriateness to culture and norms
should be prioritized and upheld for all roles (see Annex 18. Gender and polio surveillance).
2. Capacity building: While capacity building is a larger function that represents a shared responsibility
between managers and staff, it is fundamentally rooted in training. All surveillance staff should be
equipped with an initial training and advanced formal trainings,
offered either in-person or virtually, at least every two years and
with regular refresher trainings, preferably with certificates that
reference a validity period, such as an annual certification.
3. Maintaining performance: Managers should follow through on training and capacity building to
make sure field staff are supported in their roles – so their skills are applied and further developed.
• Effective supportive supervision: AFP surveillance activities must be monitored and
supervised to ensure the system remains highly sensitive. Such continuous supervision should
follow a predefined plan, using checklists for staff performance and including staff feedback and
follow-up on potential corrective actions. Regular on-the-job supportive supervision visits for
provincial and district surveillance teams should focus not on fault-finding, but on sensitization,
training, problem-solving and two-way communication. Structured tools should be used to cover
activities and present findings. Visits should review different surveillance components such as a
surveillance plan, regularly updated reporting network, an updated list of active surveillance
(AS) sites, prioritization
criteria, site visit schedule,
and site visit procedures.
Evaluating supervision is
equally important and should
be made from the national to
the province or state level,
and from the province or state
level to the district level.
• One-on-one mentoring
helps to build field staff capacity and confidence. As part of their mentoring and monitoring
roles, managers should regularly conduct active surveillance visits and case investigations with
field staff, where they can provide on-the-job demonstration and real-life examples. Ad hoc
mentoring opportunities should also be offered, based on needs.
• Managers should hold review meetings – both regular (ideally quarterly) group review
meetings and one-on-one personal reviews – to discuss performance, provide updates, and set
objectives and goals.
Six signs of effective supportive supervision
1. Surveillance officers have the appropriate technical
knowledge and skills to conduct surveillance activities.
2. Surveillance officers are – and feel – supported in their job.
3. Feedback is provided to surveillance officers.
4. Reporting procedures for cases are correctly followed.
5. Cases are investigated in a thorough and timely manner.
6. Active surveillance visits are of high quality.
A training package on AFP
surveillance is available online.
Download through POLIS

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
43
4. Staff retention: Retention among staff is bolstered when managers prioritize supportive supervision,
reward and recognize good performers, advocate for career development, add motivational inputs
during meetings (focusing on
contribution to the “big picture”), and
involve celebrities and well-known
figures to elevate the public perception
of the programme.
Staff retention is also dependent on
managers and supervisors being
sensitive and responsive to gender-
related issues. Supervisors and
managers must ensure that a gender
lens is applied to the programme both
by promoting gender equality and
addressing any gender-related barriers
or other factors that may impact the
staff safety and performance as well as
their career advancement. For more
details, see Annex 18. Gender and
polio surveillance.
Not all staff tasked with supervision are trained on supportive supervision.
Country teams should include a supervisor training that details the role and
responsibilities of supervisors. Up-to-date training modules that cover all aspects of
polio surveillance are available online and aligned with the current guidelines.
Download AFP surveillance training modules (requires POLIS access).
2. Integrating disease surveillance, the future of polio surveillance
As the world prepares for polio eradication, the WHO and other GPEI partners are actively working to
transition the polio programme to ensure key assets and capacities, including surveillance, are not lost
but successfully integrated into other programmes.
20
This will help to sustain polio surveillance within
country systems while also strengthening other surveillance programmes by building on the polio
platform where it proves beneficial.
21
Table 12 lists specific deliverables of a well-functioning AFP surveillance system that must be
maintained, as well as potential steps that can be taken to ensure integration of polio surveillance with
other programmes. These activities are foundational of AFP surveillance and must continue to support
broader, comprehensive VPD surveillance efforts, including outbreak-prone disease and syndromes.
20
Strategic Action Plan on Polio Transition. Geneva: World Health Organization; 2018 (https://www.who.int/polio-
transition/strategic-action-plan-on-polio-transition-may-2018.pdf).
21
Global strategy for comprehensive Vaccine-Preventable Disease (VPD) surveillance. Geneva: World Health Organization; 2020
(https://www.who.int/publications/m/item/global-strategy-for-comprehensive-vaccine-preventable-disease-(vpd)-surveillance).
Ways to improve supportive supervision
• Include regular (monthly or at least quarterly)
supervisory visits in workplans and plan for them as a
recurring, funded cost.
• Arrange observations in the field by accompanying staff
on a visit to a high-priority large hospital.
• Structure visits by sharing objectives, following up on
previous recommendations, and preparing updates or
refresher trainings.
• Identify gaps and generatively solve problems, making
sure to give positive feedback in public and
performance tips in private conversation.
• Openly discuss findings and recommendations.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
44
Table 12. Components of AFP surveillance that should be addressed by integration efforts
Specific deliverables
of a well-functioning
AFP surveillance
● Weekly reporting from health facilities including “zero-reporting.” Where
necessary, regular reporting from informal health service providers
● Active surveillance including physical visits of priority health facilities and
informal service providers
● Community-based surveillance in selected areas
● Active case search, if triggered by events
● Investigation of ALL AFP cases, collection of stool samples from cases and,
if indicated, AFP contact samplings, and 60-day follow-up examinations
● Testing of all stool samples at a WHO-accredited laboratory
● Meet surveillance standards at national and subnational levels
Steps that can be
taken to support
integration at the
country level
● One comprehensive surveillance operational workplan at country-level
● Core team of trained human resources at the national and subnational level
● Harmonized data collection tools and data management infrastructure
AFP = acute flaccid paralysis; WHO = World Health Organization
Resources to support integration and transition efforts
As the GPEI approaches certification, new guidance related to planning for the post-certification era will
be needed to address the latest challenges to eradication, including surveillance. All stakeholders of the
polio eradication effort are encouraged to consult the resources below.
• For the latest information, consult the GPEI website: polioeradication.org.
• For more information on transition planning, the GPEI maintains a dedicated page:
https://polioeradication.org/polio-today/preparing-for-a-polio-free-world/transition-planning,
• To support post-certification planning, the GPEI published the Polio Post-Certification Strategy
(PCS) in 2018,
22
and planning is underway to support its revision. For future updates, consult
the GPEI website: https://polioeradication.org/polio-today/preparing-for-a-polio-free-
world/transition-planning/polio-post-certification-strategy.
22
Polio Post-Certification Strategy: A risk mitigation strategy for a polio-free world. Geneva: World Health Organization; 2018
(http://polioeradication.org/wp-content/uploads/2018/04/polio-post-certification-strategy-20180424-2.pdf).
Annex guidance
Annex 19 provides further resources for GPEI programme information, as well as dedicated resources for
AFP surveillance, community-based surveillance, poliovirus laboratory testing, gender training and
surveillance for integrated VPD platforms.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
45
ANNEXES
Annex 1. Poliovirus ............................................................................................................................... 46
Annex 2. Vaccine-derived poliovirus classification and response ........................................................ 52
Annex 3. Indicators for AFP surveillance .............................................................................................. 54
Annex 4. Routine and active surveillance ............................................................................................. 60
Annex 5. Active surveillance visits ........................................................................................................ 62
Annex 6. Community-based surveillance.............................................................................................. 65
Annex 7. Examples of forms ................................................................................................................. 69
Annex 8. AFP case investigation ......................................................................................................... 77
Annex 9. Health-seeking behaviour ...................................................................................................... 81
Annex 10. Special population groups ................................................................................................... 83
Annex 11. Ad hoc active case search ................................................................................................... 86
Annex 12. AFP contact sampling .......................................................................................................... 88
Annex 13. Targeted healthy children stool sampling ............................................................................ 90
Annex 14. Rapid case and virus detection............................................................................................ 92
Annex 15. Polio committees and commissions .................................................................................... 94
Annex 16. Safety surveillance for nOPV2 ............................................................................................. 97
Annex 17. Surveillance activities in outbreak settings .......................................................................... 99
Annex 18. Gender and polio surveillance ........................................................................................... 100
Annex 19. Scientific resources ............................................................................................................ 103
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
46
Annex 1. Poliovirus
Poliovirus is a member of the enterovirus subgroup of the family Picornaviridae. Enteroviruses are
transient inhabitants of the gastrointestinal tract and are stable at an acidic pH. Picornaviruses are
small, ether-insensitive viruses with a ribonucleic acid (RNA) genome. Heat, formaldehyde, chlorine and
ultraviolet (UV) light rapidly inactivate the poliovirus.
Poliovirus has three serotypes: type 1, type 2 and type 3. All three serotypes of poliovirus cause
paralytic disease, and there is minimal heterotypic immunity between the three serotypes.
Epidemiology
Reservoir
Humans are the only known reservoir of poliovirus, which is transmitted most frequently by persons with
inapparent infection. There is no asymptomatic carrier state except in immune-deficient persons.
Transmission and temporal pattern
Poliovirus is spread by both the faecal-oral route (i.e., the poliovirus multiplies in the intestines and is
spread through the faeces) and by the respiratory route. Infection is more common in infants and young
children. Polio occurs at an earlier age among children living in poor hygienic conditions. In temperate
climates, poliovirus infections are most common during summer and autumn. In tropical areas, the
seasonal pattern is less pronounced.
The time between infection and onset of paralysis is 7–21 days. The virus spreads rapidly to non-
immune persons and transmission is usually widespread by the time of paralysis onset. The virus is
intermittently excreted for one month or more after infection. The heaviest faecal excretion of the virus
occurs just prior to the onset of paralysis and during the first two weeks after paralysis occurs.
Communicability
Poliovirus is highly infectious with seroconversion rates in susceptible household contacts of children
nearly 100% and of adults over 90%. Cases are most infectious from 7–10 days before and after the
onset of symptoms.
Immunity
Protective immunity against poliovirus infection develops by immunization or natural infection. Immunity
to one poliovirus type does not protect against infection with other poliovirus types. Immunity following
natural infection or administration of a live oral polio vaccine (OPV) is believed to be lifelong. The
duration of protective antibodies after administration of an inactivated polio vaccine (IPV) is unknown
but likely lifelong after a complete series.
23
Infants born to mothers with high antibody levels against
poliovirus are protected for the first several weeks of life.
Pathogenesis
The virus enters the body through the mouth from faecal-oral or respiratory contact. Primary
multiplication of the virus occurs at the site of implantation of the poliovirus receptor in tissues: tonsils,
intestinal cells, gut or ‘Peyer’s patches’ that line the small intestine, and lymph nodes. The virus is
usually present in the throat and in the stools before the onset of illness. One week after onset, there is
little virus in the throat, but virus continues to be excreted in the stools for several weeks. The virus
invades local lymphoid tissue, enters the blood stream, and then rarely may infect cells of the central
nervous system. The virus has “tropism” for nerve tissue and is thought to spread back along nerves
(“axons”) to the spinal cord. Replication of poliovirus in motor neurons of the anterior horn and brain
stem results in cell destruction and causes the typical manifestations of poliomyelitis in paralysis.
Paralysis extent depends on proportion of motor neurons lost. See Fig. 1.1.
23
Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hall E, Wodi AP,
Hamborsky J, et al., eds. 14th ed. Chapter 18: Poliomyelitis. Washington, D.C.: Public Health Foundation; 2021
(https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio.pdf).
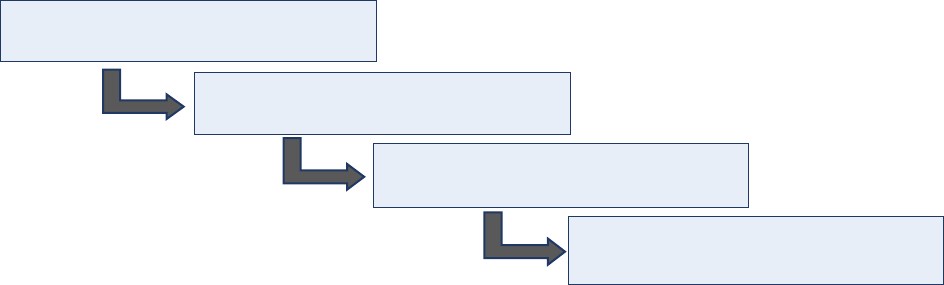
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
47
Fig. 1.1. Pathogenesis of poliomyelitis
Source: WHO.
Clinical manifestations of infection (symptoms)
The incubation period of non-paralytic poliomyelitis is 3–6 days. For the onset of paralysis in paralytic
poliomyelitis, the incubation period usually is 7–21 days (with a range from 3–35 days).
Infection with poliovirus results is a spectrum of clinical manifestations from inapparent infection to non-
specific febrile illness, aseptic meningitis, paralytic disease and death. Poliovirus infection is not
apparent in 90‒95% of infected individuals.
The following clinical pictures may present the disease (Fig 1.2):
• Abortive polio occurs as a non-specific febrile illness in 4‒8% of cases characterized by low-
grade fever, sore throat, vomiting, abdominal pain, loss of appetite and malaise. Recovery is
rapid and complete with no paralysis. It cannot usually be distinguished from other mild viral
illnesses with mild respiratory tract or gastrointestinal manifestations.
• Non-paralytic aseptic meningitis occurs in 1‒2% of infections with symptoms of headache,
neck, back and/or abdominal, extremity pain, fever, vomiting, lethargy and irritability after a
prodromal illness similar to abortive polio. Cases recover within 2‒10 days. It cannot be
clinically distinguished from other causes of aseptic meningitis.
• Paralytic poliomyelitis occurs in <1% of cases following a minor illness, sometimes separated
by several days without symptoms (biphasic). Paralytic symptoms generally begin 1–10 days
after prodromal symptoms and progress for 2–3 days. It begins with muscle pain, spasms and
return of fever, followed by rapid onset of flaccid paralysis with diminished deep tendon reflexes
that is usually complete within 72 hours. Patients do not experience sensory loss or changes in
cognition.
Depending on the sites of paralysis, poliomyelitis can be classified as spinal, bulbar or spino-bulbar
disease. Classically certain groups of muscles are affected in an asymmetrical pattern. The lower limbs
are affected more often than the upper limbs, and one leg or one part of the leg may be involved. The
affected muscles are weak and floppy (flaccid). In a very small number of cases the virus also attacks
the motor nerve cells that control the muscles of the face, throat, and tongue, and muscles of
respiration. The ability to swallow, speak and breathe becomes affected. This is known as bulbar polio
and may be fatal. Of paralytic polio cases, 2–10% are fatal due to affection of respiratory muscles, 10%
recover completely, and the remainder of cases show some residual paralysis or permanent disability.
Prognosis for recovery can usually be established within six (6) months after onset of paralytic
manifestations.
Poliovirus enters through the mouth by
faecal-oral transmission.
Virus replicates in the intestine and
lymph nodes.
Virus enters the bloodstream and
spreads to central nervous system.
The immune system responds by
releasing antibodies.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
48
Figure 1.2. Phases of occurrence of symptoms in poliomyelitis Infection
Source: WHO. Field guide for supplementary activities aimed at achieving polio eradication, Rev. 1996. Geneva: World Health
Organization; 1996;4 (https://apps.who.int/iris/bitstream/handle/10665/63478/WHO_EPI_GEN_95.01_REV.1.pdf).
Prevention
Polio vaccines provides the best protection against polio.
Poliovirus vaccines
The Global Polio Eradication Initiative (GPEI) maintains descriptions of polio vaccines.
24
1. Oral poliovirus vaccines (OPVs)
OPVs are the predominant vaccine used in the fight to eradicate polio (Table 1.1). The attenuated
poliovirus(es) contained in OPV can replicate effectively in the intestine, but it is around 10 000 times
less able to enter the central nervous system than the wild virus. This enables individuals to mount an
immune response against the virus. Virtually all countries which have eradicated polio used OPV to
interrupt person-to-person transmission of the virus.
Advantages
• OPVs are safe, effective and inexpensive, and their oral administration does not require health
professionals.
• For several weeks after vaccination, the vaccine virus replicates in the intestine, is excreted and
can be spread to others in close contact. In areas with poor hygiene and sanitation,
immunization with OPV can therefore result in “passive” immunization of people who have not
been vaccinated.
24
Global Polio Eradication Initiative. The Vaccines (webpage). (https://polioeradication.org/polio-today/polio-prevention/the-
vaccines).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
49
Disadvantages
• OPV is safe and effective. However, in extremely rare cases (at a rate of approximately 2–4
events per 1 million births),
25
the live attenuated vaccine virus in OPV can cause paralysis. In
some cases, it may be triggered by an immunodeficiency. The extremely low risk of vaccine-
associated paralytic poliomyelitis (VAPP) is well accepted by most public health programmes.
• Very rarely, when there is insufficient coverage in a community, the vaccine virus may be able
to circulate, mutate and, over the course of 12 to 18 months, reacquire neurovirulence. This is
known as a circulating vaccine-derived poliovirus (cVDPV).
Once polio has been eradicated, all OPV use will be stopped to prevent re-establishment of
transmission due to vaccine-derived polioviruses (VDPVs).
Table 1.1. Indications of use for OPVs by serotype
OPV type
Serotype
Indications for use
Monovalent oral
poliovirus vaccines
(mOPVs)
Type 1 (mOPV1)
Type 2 (mOPV2)
Type 3 (mOPV3)
Elicit the best immune response against the serotype they
target. mOPV2 is stockpiled in the event of a cVDPV2
outbreak but is progressively being replaced by nOPV2.
Novel oral polio
vaccine type (nOPV)
Type 2 (nOPV2)
Provides comparable protection against poliovirus while
being more genetically stable, therefore making it less likely
to be associated with the emergence of VDPV2 in low-
immunity settings.
At the time of writing these guidelines (2022), nOPV2 is only
being used for type 2 outbreak response under an
Emergency Use Listing of the WHO.
Bivalent oral poliovirus
vaccine (bOPV)
Type 1 and type 3
(bOPV)
Contains attenuated virus of serotypes 1 and 3. bOPV elicits
a better immune response against poliovirus types 1 and 3
than tOPV, but it does not give immunity against serotype 2.
Since April 2016, the trivalent oral poliovirus vaccine (tOPV)
has been replaced with bOPV in essential immunization and
for outbreak response against types 1 and 3 outbreaks.
Trivalent oral
poliovirus vaccine
(tOPV)
Type 1, type 2 and
type 3 (tOPV)
Withdrawn in April 2016 from essential immunization and
replaced with bOPV, tOPV can still be used in outbreak
response under specific circumstances, such as co-
circulation of type1 and type 2 polioviruses.
bOPV = bivalent oral polio vaccine; cVDPV2 = circulating vaccine-derived poliovirus type 2; mOPV = monovalent oral polio vaccine;
mOPV1 = monovalent oral polio vaccine type 1; mOPV2 = monovalent oral polio vaccine type 2; mOPV3 = monovalent oral polio vaccine
type 3; nOPV = novel oral polio vaccine; nOPV2 = novel oral polio vaccine type 2; tOPV = trivalent oral polio vaccine; VDPV2 = vaccine-
derived poliovirus type 2; WHO = World Health Organization
2. Inactivated poliovirus vaccine (IPV)
IPV consists of inactivated (killed) poliovirus strains of all three poliovirus types. IPV is given by
intramuscular or intradermal injection and as such needs to be administered by a trained health worker.
It produces antibodies in the blood to all three types of polioviruses. In the event of infection, these
antibodies prevent the spread of the virus to the central nervous system and protect against paralysis.
25
This rate is expected to significantly decline, as the type 2 component of oral polio vaccine was removed from essential
immunization worldwide in April 2016; this type was responsible for approximately 40% of all VAPP cases.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
50
IPV is used in essential immunization and, in some instances, in outbreak response. As IPV does not
stop transmission of the virus, OPV is the vaccine of choice for outbreak response activities even in
countries that rely exclusively on IPV for their essential immunization programmes.
Advantages
• As IPV is not a ‘live’ vaccine, it carries no risk of VAPP. It is one of the safest vaccines in use.
• IPV triggers an excellent protective immune response in most people.
Disadvantages
• IPV induces very low levels of immunity in the intestine. As a result, when a person immunized
with IPV is infected with wild poliovirus, the virus can still multiply inside the intestines and be
shed in the faeces, thereby risking continued circulation.
• Administering the vaccine requires trained health workers, as well as sterile injection equipment
and procedures.
• IPV is over five times more expensive than OPV.
Laboratory diagnosis
Poliovirus isolation in culture is the most sensitive method to diagnose poliovirus infection. Poliovirus is
most likely to be isolated from stool specimens. It may also be isolated from pharyngeal swabs.
Isolation is less likely from blood or cerebral spinal fluid.
To increase the probability of isolating poliovirus, two stool specimens are collected 24 hours apart from
patients with suspected poliomyelitis, ideally within 14 days after onset.
Real-time reverse transcription polymerase chain reaction (RT-PCR) is used to differentiate possible
wild strains from vaccine-like strains (“intratypic differentiation”), using virus isolated in culture as the
starting material.
Molecular techniques are done to fully characterize the poliovirus. Maintaining a reference bank of the
molecular structure of known viruses allows the geographic origin of new isolates to be traced.
Differential diagnosis
The differential diagnosis of acute flaccid paralysis (AFP) includes paralytic poliomyelitis, Guillain-Barré
syndrome (GBS) and transverse myelitis. Less common etiologies are traumatic neuritis, encephalitis,
meningitis, other enterovirus infections and tumours (Table 1.2).
Distinguishing characteristics of paralytic polio are asymmetric flaccid paralysis, fever at onset, rapid
progression of paralysis, residual paralysis after 60 days and preservation of sensory nerve function.
Clinical case management
There is no specific treatment for poliomyelitis. Suspected AFP cases should be referred to a hospital
immediately for medical care. Any problem with respiration suggesting involvement of the diaphragm
requires immediate attention. Supportive care should be given to paralytic cases under physician
management.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
51
Table 1.2. Differential diagnosis of poliomyelitis
Key features
Poliomyelitis
Guillain-Barré
syndrome
Traumatic neuritis
Transverse myelitis
Progression of
paralysis
24–72 hours onset to
full paralysis
From hours to 10 days
From hours to 4 days
From hours to 4 days
Fever at onset
High, always present
at onset of flaccid
paralysis, gone the
following day
Not common
Commonly present
before, during, and
after flaccid paralysis
Rarely present
Flaccid paralysis
Acute, usually
asymmetrical,
principally proximal
Generally acute,
symmetrical and distal
Acute, asymmetrical
and affecting only one
limb
Acute, lower limbs,
symmetrical
Muscle tone
Reduced or absent in
affected limb
Global hypotonia
Reduced or absent in
affected limb
Hypotonia in lower
limbs
Deep-tendon reflexes
Decreased to absent
Globally absent
Decreased to absent
Absent in lower limbs
early, hyperreflexia late
Sensory symptoms
and sensation
Severe myalgia,
backache, no sensory
changes
Cramps, tingling,
hypoesthesia of palms
and soles
Pain in gluteus,
hypothermia
Anaesthesia of lower
limbs with sensory
level
Cranial nerve
involvement
Only when bulbar
involvement is present
Often present,
affecting nerves VII, IX,
X, XI, XII
Absent
Absent
Respiratory
insufficiency
Only when bulbar
involvement is present
In severe cases,
enhanced by bacterial
pneumonia
Absent
Sometimes
Autonomic signs
and symptoms
Rare
Frequent blood
pressure alteration,
sweating, blushing,
body temperature
fluctuations
Hypothermia in
affected limb
Present
Cerebrospinal fluid
Inflammatory
Albumin-cytologic
dissociation
normal
Normal or mild in cells
Bladder dysfunction
Absent
Transient
Never
Present
Nerve conduction
velocity: third week
Abnormal: anterior
horn cell disease
(normal during the first
two [2] weeks)
Abnormal: slowed
conduction, decreased
motor amplitude
Abnormal: axonal
damage
Normal or abnormal,
no diagnostic value
Electromyography
(EMG) at three weeks
Abnormal
Normal
Normal
Normal
Sequelae at two (2)
months and
up to a year
Severe, asymmetrical
atrophy, skeletal
deformities developing
later
Symmetrical atrophy of
distal muscles
Moderate atrophy, only
in affected lower limb
Flaccid diplegia,
atrophy after years
Sources: WHO. Field guide for supplementary activities aimed at achieving polio eradication, Rev. 1996. Geneva: World Health
Organization; 1996;4 (https://apps.who.int/iris/bitstream/handle/10665/63478/WHO_EPI_GEN_95.01_REV.1.pdf). Marx A, Glass JD,
Sutter RW. Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiol Rev 2000;22(2):298-316
(https://doi.org/10.1093/oxfordjournals.epirev.a018041).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
52
Annex 2. Vaccine-derived poliovirus classification and response
There are three categories of vaccine-derived polioviruses (VDPVs), each with a unique classification
and associated mode of response (Fig. 2.1).
Circulating vaccine-derived poliovirus (cVDPV): Through serial transmission of vaccine virus in an
undervaccinated community, the attenuated polioviruses can regain neurovirulence and transmission
characteristics of wild poliovirus (WPV). VDPVs that have been established through community
circulation in undervaccinated populations are classified as circulating vaccine-derived polioviruses
(cVDPVs). These have become an urgent issue for the polio eradication programme as cVDPVs have
been responsible for thousands of poliomyelitis cases since their first characterization in 2000.
26
Strengthening essential immunization systems and conducting supplemental immunization activities
(SIAs) are necessary to avoid an emergence of cVDPV. After community transmission has become
established, interrupting cVDPV requires outbreak response measures, including high-quality SIAs to
reach every child in affected communities.
27
Immunodeficiency-associated vaccine-derived poliovirus (iVDPV): A far smaller but potentially
serious challenge to sustaining global polio eradication is represented by VDPVs that evolve in and are
excreted by patients with inherited primary immunodeficiency disorders (PIDs) affecting the B-cell
system. Following exposure to oral polio vaccine (OPV) viruses, PID patients may shed
immunodeficiency-associated vaccine-derived polioviruses (iVDPVs) that can cause paralytic polio for
the individual and can re-establish transmission within the community. Infected PID patients may shed
iVDPV for months or years before the patient becomes paralysed or before the virus they shed initiates
community circulation. To mitigate the individual and community risks posed by iVDPVs during the polio
endgame and the post-eradication era, iVDPV surveillance will be important. Once country programmes
identify non-paralytic PID patients excreting polioviruses, iVDPV surveillance provides strategies and
treatments to rid both the individual and the community of the risk posed by iVDPVs.
28
Ambiguous vaccine-derived poliovirus (aVDPV): A final category of poliovirus is the ambiguous
vaccine-derived poliovirus (aVDPV), termed “ambiguous” because these viruses cannot be genetically
linked to previously known VDPVs and because the individuals excreting the virus do not have a known
immunodeficiency. aVDPVs may be an early indication of the possibility of a cVDPV developing, and
therefore surveillance needs to be ramped up as soon as one is detected.
26
Public Health Dispatch: Outbreak of Poliomyelitis --- Dominican Republic and Haiti, 2000. MMWR Morb. Mortal. Wkly. Rep.
2000;49(48);1094,1103 (https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4948a4.htm).
27
Global Polio Eradication Initiative (GPEI). Standard operating procedures: responding to a poliovirus event or outbreak, version
4. Geneva: World Health Organization; 2022 (https://polioeradication.org/wp-content/uploads/2022/07/Standard-Operating-
Procedures-For-Responding-to-a-Poliovirus-Event-Or-Outbreak-20220807-EN-Final.pdf).
28
Global Polio Eradication Initiative (GPEI). Guidelines for Implementing Poliovirus Surveillance among Patients with Primary
Immunodeficiency Disorders (PIDs), revised 2022. Geneva: World Health Organization; 2022 (https://polioeradication.org/wp-
content/uploads/2022/06/Guidelines-for-Implementing-PID-Suveillance_EN.pdf).

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
53
Fig. 2.1. Classification Iand response to reported VDPV isolates
Note: Note that the classification of VDPV isolates is done by the sequencing laboratory in collaboration with the WHO regional polio team.
aVDPV = ambiguous vaccine-derived poliovirus; cVDPV = circulating vaccine-derived poliovirus; iVDPV = immunodeficiency-associated
vaccine-derived poliovirus; PID = primary immunodeficiency disorder; VDPV = vaccine-derived poliovirus
Source: GPEI. Classification and reporting of vaccine-derived polioviruses (VDPV). Geneva: World Health Organization; 2016
(https://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf).
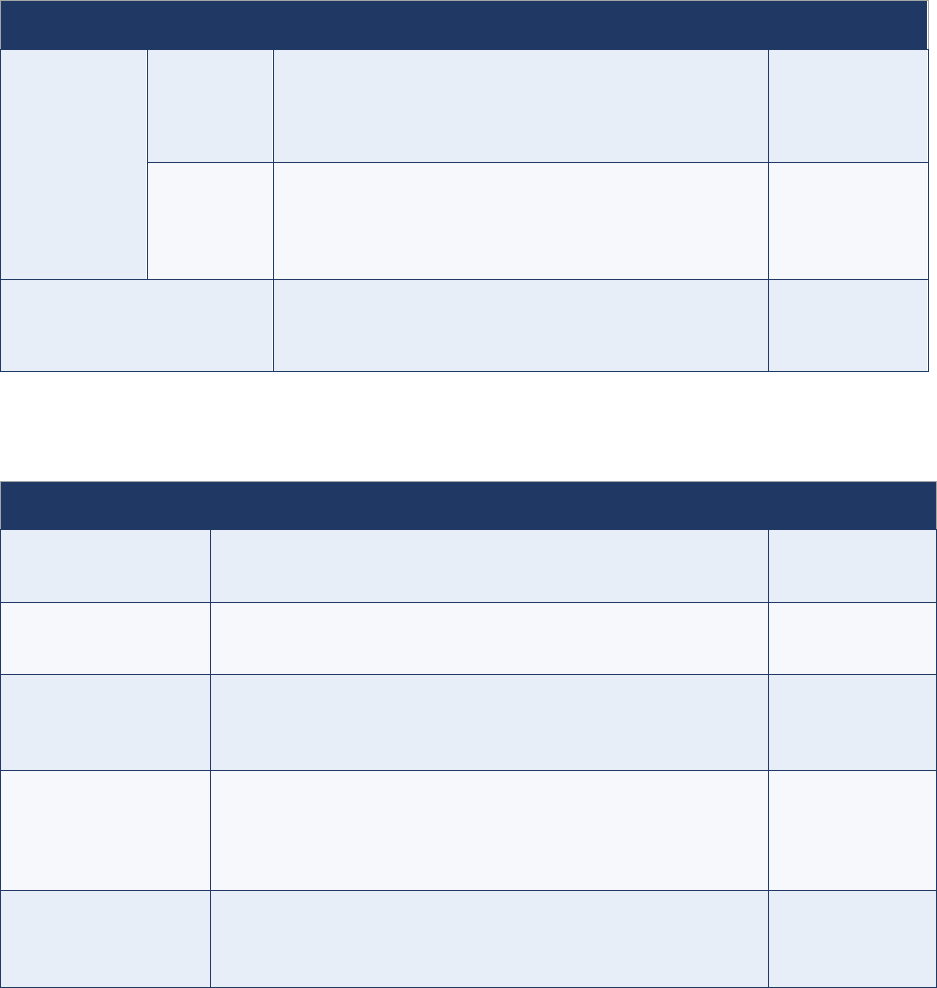
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
54
Annex 3. Indicators for AFP surveillance
Indicators highlighted in bold are monitored at the country, regional and global levels; indicators that
are not bolded are monitored at the regional and/or country levels only.
Core indicators on timeliness
Core indicators on timeliness, as introduced by the GPEI 2022-2026 Strategy, capture the overall
capacity of the programme to identify rapidly any wild poliovirus (WPV) or vaccine-derived poliovirus
(VDPV). This capacity has been defined as: (1) the capacity of the programme to report a positive acute
flaccid paralysis (AFP) case rapidly so that a response can be mounted fast; and (2) the capacity to
process rapidly any positive specimen (Table 3.1). Additional indicators highlight the capacity of the
programme to report any laboratory results rapidly, regardless of the final result.
Table 3.1. Overall indicators on timeliness
Indicator
Calculation (expressed as a percentage)
Target
Overall
detection of
WPV/VDPV
For AFP (1)
# of AFP cases* with WPV/VDPV final lab results
<=35 days of onset
/
# of AFP cases* with WPV/VDPV final lab results
>=80%
System
capacity (2)
†
# of WPVs and VDPVs with final lab results <=35 days of
onset for AFP cases
/
# of WPVs and VDPVs
>=80%
AFP detection – system
# of AFP cases* with final lab results <=35 days of onset
/
# of AFP cases*
>=80%
AFP = acute flaccid paralysis; VDPV = vaccine-derived poliovirus; WPV = wild poliovirus
*Aggregated results: all lab results (AFP + contacts) used to classify AFP case as confirmed/discarded.
†
Specimen-based calculation
Table 3.2. Indicators on timeliness for field activities
Indicator
Calculation (expressed as a percentage)
Target
Timeliness of
notification
# of AFP cases reported <=7 days of onset
/
# of AFP cases
>=80%
Timeliness of
investigation
# of AFP cases investigated <=48 hours of notification
/
# of AFP cases
>=80%
Timeliness of field
activities
# of AFP cases with 2 stool specimens collected >=24 hrs apart
AND <=11 days of onset
/
# of AFP cases
>=80%
Timeliness of field
and shipment
activities
# of AFP cases with 2 stool specimens collected >=24 hours
apart AND received in good condition* at a
WHO-accredited laboratory AND <=14 days of onset
/
# of reported AFP cases
>=80%
Timeliness of stool
specimen shipment
# of stool specimens that arrive in good condition* at a WHO-
accredited lab AND <=3 days of specimen collection
/
# of stool specimens collected
>=80%
AFP = acute flaccid paralysis; WHO = World Health Organization
*For calculations: missing stool condition = poor condition
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
55
Table 3.3. Indicators on timeliness for laboratory activities
Indicator
Calculation (expressed as a percentage)
Target
AFP: Timeliness of
reporting laboratory
results (system
performance)
# of stool specimens with final lab results available <=21 days from a
direct detection country OR <=28 days from a non-direct detection
country of receipt at a WHO-accredited lab
/
# of stool specimens collected
>=80%
AFP: Timeliness of
reporting WPV/VDPV
results (detection)
# of stool specimens with WPV/VDPV final lab results available <=21
days of receipt from a direct detection country OR <=28 days of receipt
from a non-direct detection country at a WHO-accredited lab
/
# of stool specimens collected positive for WPV/VDPV
>=80%
AFP: Timeliness of
reporting poliovirus
laboratory results
# poliovirus stool specimens with sequencing results available <=7
days of receipt at a WHO-accredited sequencing lab
/
# of PV stool specimens positive by ITD
requiring sequencing
>=80%
AFP = acute flaccid paralysis; ITD = intratypic differentiation; VDPV = vaccine-derived poliovirus; WHO = World Health Organization;
WPV = wild poliovirus
Core indicators on surveillance quality
Table 3.4. Core indicators on AFP surveillance quality
Indicator
Calculation
Target
NPAFP rate*
(# of cases discarded as NPAFP in children <15 years of age
/
# of children <15 years of age)
x
100 000 per year
Note: Endemic countries are encouraged to have >=3
AFR, EMR, SEAR: >=2
AMR, EUR, WPR: >=1
OB-affected:
†
>=2
NPAFP rate –
subnational
(# of districts with >=100 000 children <15 years old that meet the
NPAFP rate target
/
# of districts with >=100 000 children <15 years old)
x
100
Note: Need to reach >=3 per 100,000 in all high-risk districts within
an outbreak country
AFR, EMR: >=80%
SEAR: >=50%
AMR, EUR, WPR: NA
OB-affected districts:*
100%
Stool adequacy
(# of AFP cases with 2 stool specimens collected >=24 hours apart
AND <=14 days of onset AND received in good condition‡ in a
WHO-accredited laboratory
/
# of AFP cases)
x
100
Note: Certification indicator (14 days)
≥80%
AFP = acute flaccid paralysis; AFR = African Region; AMR = Region of the Americas; EMR = Eastern Mediterranean Region;
EUR = European Region; NA = not applicable; NPAFP = non-polio acute flaccid paralysis; OB = outbreak; SEAR = South-East Asia
Region; WHO = World Health Organization; WPR = Western Pacific Region
*Rate should be annualized.
†Outbreak-affected country is defined as: any country experiencing an outbreak of WPV or circulating vaccine-derived poliovirus (cVDPV)
currently or in the previous 12 months.
‡For calculation: missing stool condition = poor condition
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
56
Table 3.4 (continued)
Indicator
Calculation (expressed as a percentage)
Target
Stool adequacy –
subnational
(# of districts that reported >=5 AFP cases that meet the stool
adequacy target
/
# of districts that reported >=5 AFP cases)
x
100
≥80%
Stool timeliness
(# of AFP cases with 2 stool specimens collected >=24 hrs
apart, AND <=14 days of onset
/
# of reported AFP cases)
x
100
Note: Certification indicator (14 days of onset)
≥80%
Stool condition
# of AFP cases with two stool specimens arriving in good
condition
*
at a WHO accredited lab
/
# of reported AFP cases
>=80%
Composite index –
national
Population living in districts that meets both NPAFP rate target
and stool adequacy target
/
Population living in all districts (Admin2)
>=80%
Composite index –
subnational
# of districts with ≥100,000 children <15 years old that meet
NPAFP rate target and stool adequacy target
/
# of districts with ≥100,000 children < 15 years of age
>=80%
Adequacy of active
surveillance visits
†
(2 calculations)
1. # visits to HP sites conducted / # HP site visits planned
2. # HP sites visited / Total # HP sites
1. >=80%
2. 100%
Completeness of 60-
day follow-ups
# of inadequate AFP cases with a follow up exam for residual
paralysis completed >=60 days AND <= 90 days of onset
/
# of inadequate AFP cases
>=80%
Completeness of
weekly zero reporting
(WZR)
# of sites reporting
/
# of designated reporting sites for AFP surveillance
>=80%
Timeliness of WZR
# of sites reporting by the deadline
/
# of designated reporting sites for AFP surveillance
>=80%
AFP = acute flaccid paralysis; HP = high-priority; NPAFP = non-polio acute flaccid paralysis; WZR = weekly zero reporting
*
For calculation: missing stool condition = poor condition
†
(a) High-priority sites are those facilities where there is a high likelihood of seeing an AFP case; they are visited at least on a weekly basis
and sometimes more often, (b) Combination indicator in which “all HP sites have >=1 visit each month” to be used as a flag, (c) Calculated
per month.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
57
Non-core indicators
Table 3.5. Non-core indicators on AFP surveillance*
†
Indicator
Calculation (expressed as a percentage)
Target
Unreported AFP cases found
during active surveillance
# of unreported AFP cases found in the register during
active surveillance visits
/
month
None
Percentage of supervised active
surveillance visits
‡
# of active surveillance visits supervised per month
/
# of active surveillance visits conducted per month
>=25%
Number of supervisory visits
in high-priority sites
# HP sites with >=1 supervised visit in the last 6 months
/
# of HP sites
100%
AFP case field validation
Note: as opposed to a clinical validation;
would be done by a supervisor or higher than
the person who reported the case
# of AFP cases validated <=14 of investigation
/
# of AFP cases
>=30%
Completeness of
AFP contact sampling
# of inadequate AFP cases with contact sampling
§
/
# of inadequate AFP cases
>=80%
Timeliness of
AFP contact sampling
# of contact stool specimens of inadequate cases
collected <=7 of days of investigation
/
# of contact stool specimens of inadequate cases
>=80%
AFP = acute flaccid paralysis; HP = high-priority
* For priority countries (very high risk, high risk, and medium-high risk), indicators should be analysed monthly.
†
For non-priority countries, indicators should be reviewed quarterly and included in desk reviews.
‡
Calculated by priority site, by geography, and by quarter.
§
2 or 3 contact samples per inadequate AFP case, as per regional recommendation.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
58
Table 3.6. Non-core indicators on health-seeking behaviours
*†
Indicator
Calculation (expressed as a percentage)
Target
AFP case encounters
‡
# of AFP cases with <=2 health encounters
between onset and notification
/
# of AFP cases
>=80%
Adequacy of notification by
designation
# of 1st health encounters that led to a notification,
by designation [reporting source]
§
/
# of health encounters by that same designation
>=80%
Appropriateness of
surveillance network
# of AFP cases with first health encounters with a reporting site
within the AFP surveillance network
/
# of AFP cases
>=80%
Late reported AFP cases:
Completeness of health
encounter information
Among AFP cases reported >14 days after paralysis onset:
# of AFP cases with no information on health encounters
/
# AFP cases reported >14 days after paralysis onset
>=80%
AFP = acute flaccid paralysis
* For priority countries (very high risk, high risk, medium-high risk), indicators should be analysed monthly.
†
For non-priority countries, indicators should be reviewed quarterly and included in desk reviews.
‡
Results should be stratified by sex.
§
This is the “percentage of 1
st
encounters by designation (e.g., doctor, nurse, traditional healer, vaccinator, other) that led to the
notification of an AFP case.”
Table 3.7. Non-core indicators on community-based surveillance
Indicator
Calculation (expressed as a percentage)
Target
Proportion of AFP cases
reported by CBS
# of AFP cases (those on linelist) identified by community
informant
/
# of AFP cases on linelist
TBD
Proportion of ‘verified’ AFP
reported by CBS
# of ‘suspect’ AFP cases identified by community informant
/
# of AFP cases ‘verified’ by surveillance officers
TBD
Completeness of
weekly/monthly zero reporting
(WZR/MZR)
# of reports received from community informants
/
# of expected reports from community informants
>=80%
Timeliness of WZR/MZR
# of reports received on time from community informants
/
# of expected reports from community informants
>=80%
Proportion of female informants
# of female informants
/
# of informants
>=50%-80%*
Proportion of informants from
local area
# of local informants
/
# of informants
>=80%*
AFP = acute flaccid paralysis; CBS = community-based surveillance; MZR = monthly zero reporting; TBD = to be determined;
WZR = weekly zero reporting
*Target to be adjusted at the country level; priority countries to regularly analyse.
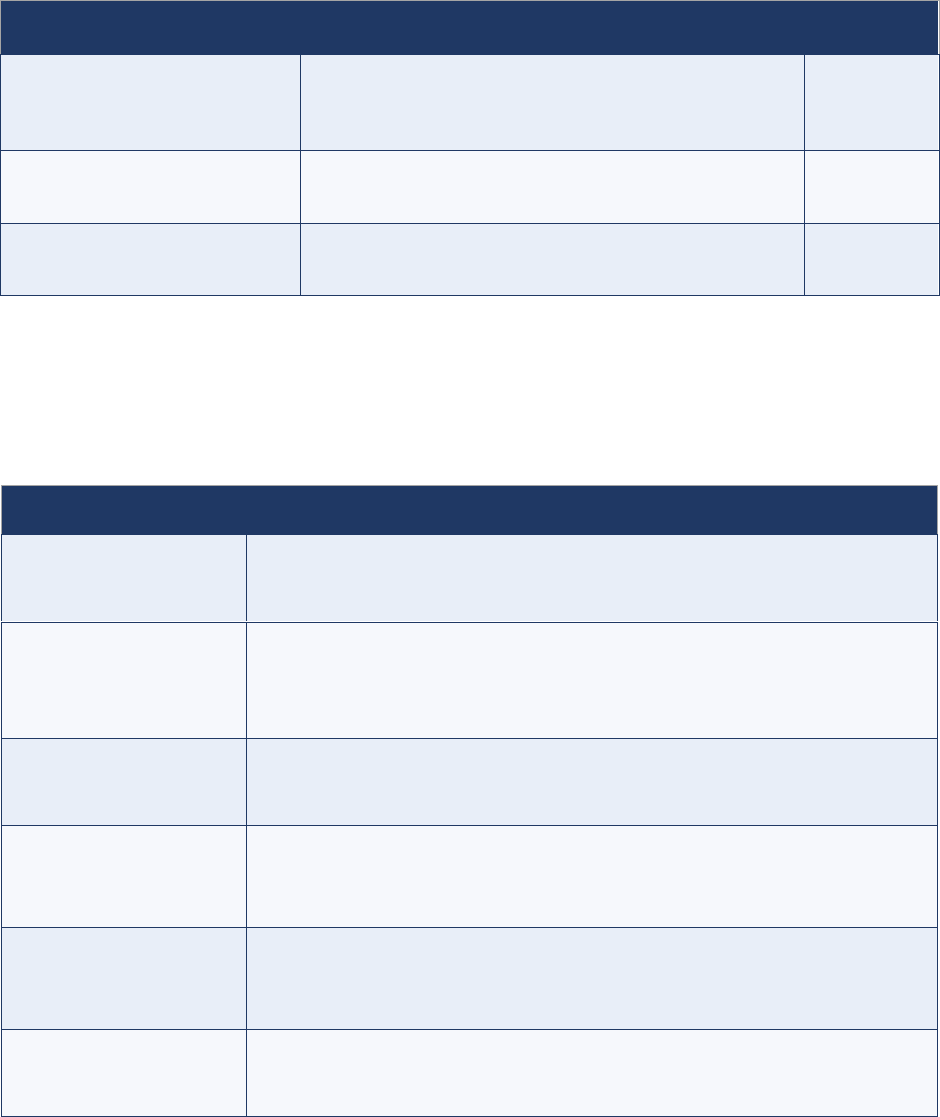
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
59
Table 3.7 (continued)
Indicator
Calculation (expressed as a percentage)
Target
Supervision of informants
† ‡
# of informants who have received at least one supervisory
visit in last 3 months
/
# of informants
>=80%
Informant training
‡ §
# of informants with training within the last year
/
# of informants
>=80%
Informant turnover rate
‡ § ¶
# of informants who left during the previous year
/
# of informants
TBD
† To be reviewed quarterly; priority countries to regularly analyse. Suggest to stratify results by supervisor.
‡ Results should be stratified by sex.
§ To be reviewed annually; priority countries to regularly analyse.
¶ Informant turnover rate is a flag; the target is to be defined at the country level. The calculations should be based on the number of
informants at the beginning of the review period.
Table 3.8. Gender-related indicators
Indicators
Calculation (expressed as a percentage)
Case detection
# of AFP cases* by sex with final lab results ≤35 days of onset
/
# of AFP cases
Timeliness of field
activities
# of AFP cases by sex with 2 samples collected ≥ 24 hrs apart, both within 11 days
of paralysis onset
/
# of reported AFP cases
Timeliness of notification
# of AFP cases by sex reported within 7 days of paralysis onset
/
# of reported AFP cases
Health contact
# of AFP cases by sex with ≤2 healthcare encounters between onset and before
notification
/
# of AFP cases
Professional profile by
sex (by category)
# of women [professional profile]
/
total # of staff or informants (by category: surveillance officer, supervisor, CBS
informant)
Staff with
completed PRSEAH
# of surveillance staff having completed PRSEAH training
/
# of staff
AFP = acute flaccid paralysis; CBS = community-based surveillance; PRSEAH = preventing and responding to sexual exploitation, abuse
and harassment
*Aggregated results: all lab results (AFP + contacts) used to classify AFP case as confirmed/discarded
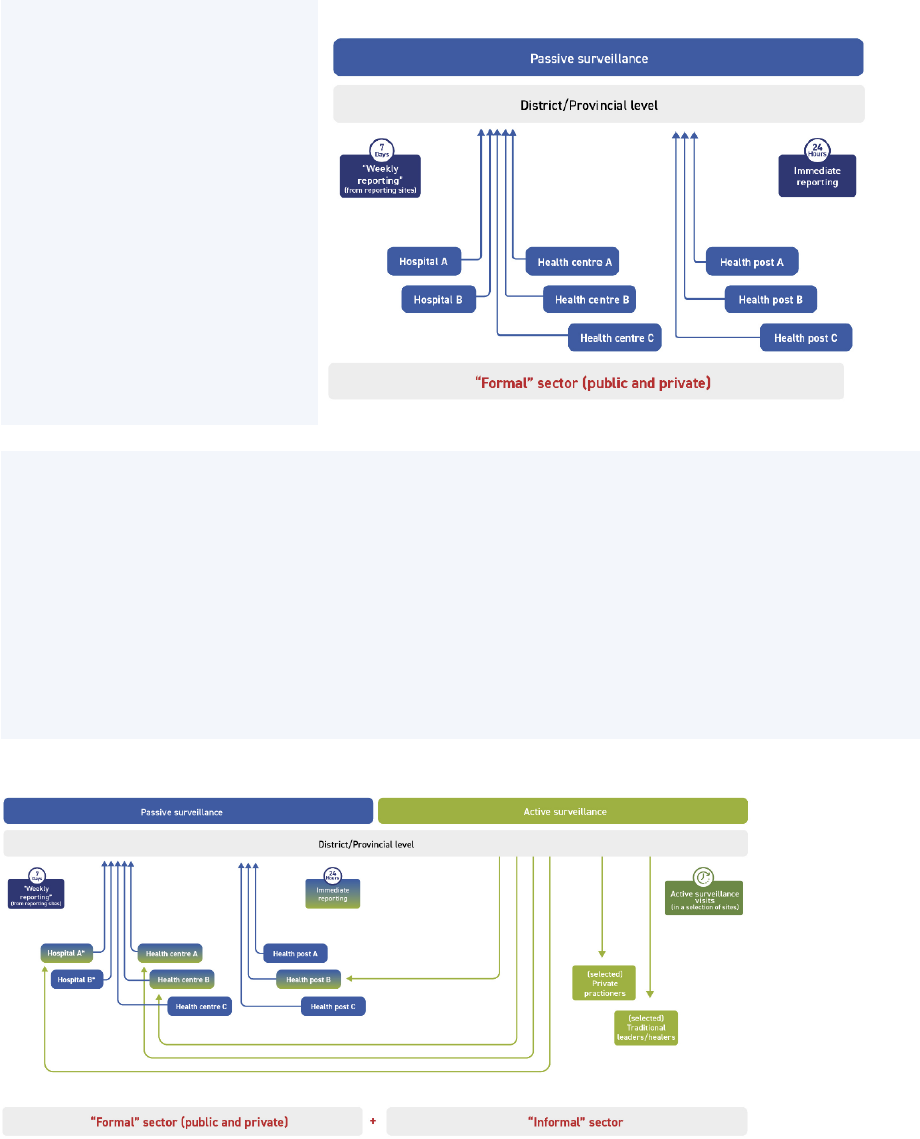
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
60
Annex 4. Routine and active surveillance
Field reviews of acute flaccid paralysis (AFP) surveillance have shown that the difference between
routine (passive) and active surveillance is not sufficiently clear in many countries. At the most basic
level, routine surveillance relies on “reports being sent,” while active surveillance (AS) is the process of
“surveillance staff going physically to visit health facilities” (Figs. 4.1 and 4.2). While the AS network
includes routine surveillance sites that report on AFP, the activity of prioritizing, scheduling and
conducting AS visits to actively search for AFP cases in facility records distinguishes AS from routine
surveillance (Fig. 4.3)
A. Routine surveillance
Fig. 4.1. Representation of routine (passive) surveillance
• All facilities that are part
of the routine (passive)
surveillance network
(“reporting sites”) should
immediately notify any
AFP case they see to the
district / provincial level.
• All facilities should also
send weekly and/or
monthly reports to the
district / provincial level
(blue arrows).
Source: WHO.
B. Active surveillance (AS)
• Reporting sites in the formal sector that are most likely to see AFP cases are selected for AS
(blue-green boxes).
• Informal sector actors (not in passive surveillance reporting) are engaged for AS because of their
likelihood of seeing AFP cases (green boxes).
• All AS sites, whether formal or informal, should also notify an AFP case immediately.
• District and provincial surveillance teams regularly visit all AS sites (green arrows).
Within hospitals, AS visits should be conducted in wards that are likely to see AFP cases: paediatric
wards, internal medicine, inpatient, outpatient, emergency, etc.
Fig. 4.2. Representation of active surveillance
Source: WHO.
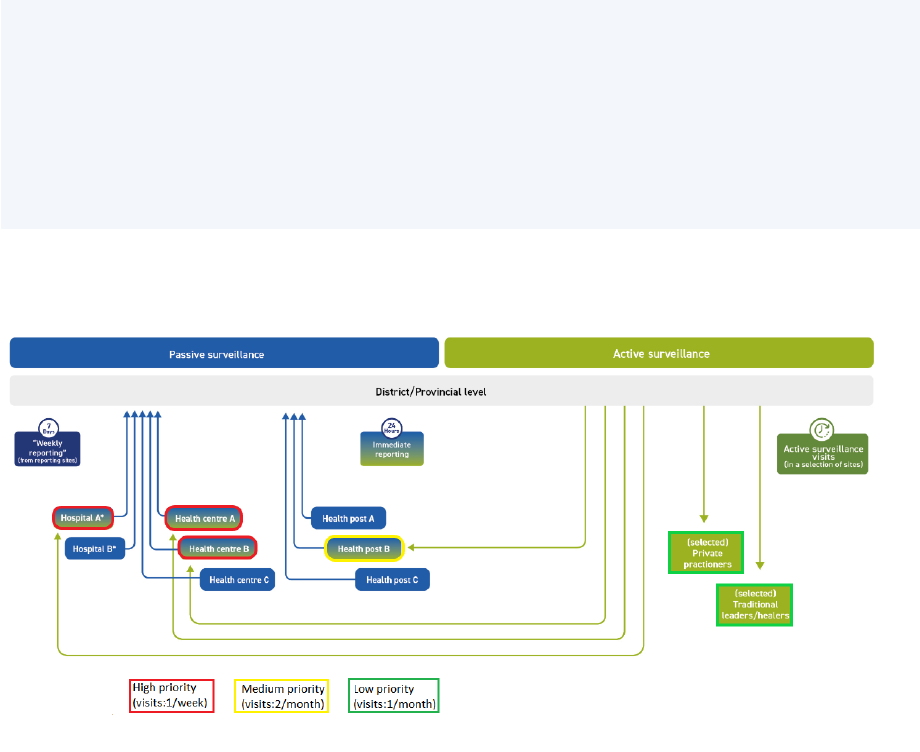
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
61
C. Prioritizing AS sites
The sites with the highest likelihood of seeing an AFP case should be prioritized over other sites. This
could include large hospitals with a paediatric ward or a medium-sized health centre in a province. The red
boxes highlight high-priority sites; yellow boxes, medium-priority sites, and green boxes, low-priority sites.
The frequency of AS site visits depends on the priority of the facility with high-priority sides often visited
weekly or twice a week, medium-priority sites visited every two weeks or monthly, and low-priority sites
visited monthly or quarterly. The frequency must be adjusted based on the local epidemiological context.
Fig. 4.3. Representation of approaches to AS site prioritization
Source: WHO.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
62
Annex 5. Active surveillance visits
The World Health Organization (WHO) has published guidance for active surveillance
(AS) that includes tips on making the best use of surveillance sites and informants and for
improving the overall sensitivity of active surveillance for acute flaccid paralysis (AFP).
Download “Best practices in active surveillance for polio eradication.”
Steps in conducting active surveillance (AS) visits
Before you leave your office
1. Make sure you have:
✓ stool collection kits
✓ case investigation forms
✓ the most recent AFP line list
✓ communications material (e.g., posters)
✓ notebook and pen
✓ tape and thumbtacks (to put up posters or case definitions)
When you arrive at the AS site
2. Meet with the facility surveillance focal person. (Note: If this is your first visit to the site, pay a
courtesy visit to the director of the facility to explain the purpose of your visit and ask permission to
conduct regular visits.)
3. Ask the surveillance focal person if the site has received or seen a case meeting the definition of
AFP since the last visit.
4. Conduct a case search by:
✓ visiting the children’s wards and
specialized services (e.g., orthopaedics,
rehabilitation centres); and
✓ checking the patient register(s) in the
inpatient, outpatient, emergency and
paediatrics departments for any
preliminary or final diagnosis of disease or
condition that could have caused an AFP.
If no diagnosis, look for signs or
symptoms. Do this for all visits since the
last visit.
5. Collect in your notebook the names and
addresses of AFP cases you find.
6. In the register, note the result of your search
below the last registered patient (number of
AFP cases found in the register, e.g., “0 AFP
cases found,” if none found) with today’s
date. Add your signature, so that supervisors
will know that you have visited.
Active surveillance is detective work
Records rarely indicate diagnoses. If there is
a polio case, you may not find “polio” or
“poliomyelitis” in health records. Furthermore,
signs and symptoms described will rarely
correspond to the AFP case definition.
Some words and phrases you might see:
• Paralysis, paresis (weakness), flaccid (soft)
• Weakness, hypotonia of a limb, weakness
of unknown origin
• Frequent falls, walking distortion
• “Can no longer walk”
• “Can no longer stand up”
Keep in mind:
These can be in any language or dialect.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
63
7. If you find a case in the register that looks like a missed AFP case, ask whether this case was
already reported. Also, compare it to the national AFP line list.
8. If you establish that the case is “new” – that is, not previously reported – plan to investigate it as
soon as possible.
9. Sensitize the surveillance focal person, if new to
the job, and other people likely to encounter a
case, such as nurses, if they’re not familiar with
AFP surveillance. (Note: If the facility has no
surveillance focal point yet, for example if it is a
new site, make sure that a focal point is identified
and trained.) See Table 5.1 for a summary of
focal point responsibilities.
10. Give feedback on the facility’s “zero reports”
(routine reporting), if necessary (i.e., in case of
incomplete or late reports).
11. Provide the site with:
• AFP case investigation forms and stool
collection kits for high-priority sites; and
• case definitions, posters, flyers, etc., for all sites.
If possible, put up the case definitions and posters yourself.
12. Thank the staff and remind them of the date of your next visit.
Note: If a country is implementing integrated surveillance, the AS visit will cover several diseases and
may also involve checking the vaccine stock and cold chain. Officers conducting AS visits should
receive training to build their capacities on those integrated activities. AS forms are usually modified to
reflect integration of disease surveillance with other vaccine-preventable diseases (VPDs).
After you return to the district office
11. Note the salient results of the visit in the supervisory notebook (including people met and
sensitized, weaknesses observed, number of cases found) for your record and reports.
12. Immediately notify any new AFP case(s) to the national level and launch AFP case
investigations.
Experience has shown that suitable focal points vary by facility.
● In smaller hospitals, it may be the person already designated for reporting notifiable
diseases or sending the weekly or monthly routine report.
● In larger hospitals, routine reporting is often carried out by an experienced nurse or
infection control nurse; however, a clinician may also be designated.
● In hospitals with paediatric departments, paediatricians actively involved in managing
patients in the emergency department or paediatric wards (not necessarily the chief of
the paediatric department) should be designated as facility focal point.
Communicating with focal points
• With clinicians, “I’m looking for AFP
cases, not polio. There will be no
additional work for you.”
• With traditional practitioners and
midwives, “Your patients will remain your
patients. There is no competition, and all
test results will be shared with you.”
• With refugee camps and at entry points,
“Here’s an AFP case definition, which is
the purpose of my visit.”

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
64
Table 5.1. Focal point responsibilities for active surveillance
Responsibility
Related duties
Immediate notification of
an identified AFP case
and case investigation
support
● Whenever a doctor or nurse in an AS site encounters a patient with
AFP, the designated AFP focal point should be immediately informed.
● The AFP focal point without delay should contact the responsible
district or province surveillance team to report the AFP case.
● The AFP focal point may initiate stool collection.
● The AFP focal point will liaise with and lend support to public health
staff or surveillance officers who arrive to conduct an AFP case
investigation, to include gathering pertinent information.
Coordination with public
health staff during AS visits
● The AFP focal point is the primary contact for public health staff
visiting regularly to conduct AS visits.
● During each visit, the public health officer will contact the AFP focal
point to ask whether cases have been seen and discuss recently
reported cases.
Confirmation of zero
reporting
● Before a routine report is sent, the AFP focal point must make sure
that sending a “zero report” means no AFP case was seen in the
facility during the reporting period.
AFP = acute flaccid paralysis; AS = active surveillance
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
65
Annex 6. Community-based surveillance
Needs assessment
Before implementing community-based surveillance (CBS), a needs assessment must first be carried
out and other potential surveillance strengthening options must be explored.
The needs assessment is a situational analysis that explores the following questions:
• How well does the current acute flaccid paralysis (AFP) surveillance system cover special
populations or hard-to-reach areas?
• What are the real issues behind surveillance gaps? Are they related to healthcare access and
utilization or cultural acceptability?
• Is the current system an event-based surveillance (EBS) where polio is one of the signals?
• Who are primary reporters of AFP cases in the community? Are they included in the AS network?
• Are CBS activities currently operating for other diseases?
• Is linking informants to existing health facilities an option?
• What are the health-seeking behaviours of the communities and what are the influencing factors?
(e.g., gender, ethnicity, internally displaced population (IDP) or refugee, place of residence, etc.)
• What resources in the area should be consulted, such as healthcare facilities and providers (public
and private), humanitarian agencies (United Nations [UN], etc.), and nongovernmental
organizations (NGOs)?
• What healthcare providers and existing community networks, particularly women’s groups,
professional and political networks, and grassroots organizations, could be engaged?
Process to establish CBS
If the conclusion of the needs assessment is that CBS is the most effective strategy to improve AFP
surveillance sensitivity and no other surveillance strategies can deliver for a specific population or area,
the process to establish CBS is to decide on the modality and follow the process below.
CBS generally has two modalities:
• Formal CBS has a high resource intensity with incentives, close supervision, and
telecommunication tools (e.g., auto-visual AFP detection and reporting, or AVADAR). It usually
functions independently of the facility-based surveillance with informants directly linked with
surveillance officers.
• Informal CBS has a low-resource intense modality with volunteers or informants sensitized
annually and receiving minimal incentives for reporting verified true AFP cases. Informants are
usually linked to focal points within nearby health facilities, so informal CBS often works more
closely with facility-based surveillance.
The process to establish CBS involves the following steps:
1. Sensitization: Identify, sensitize and brief key community actors (local and religious leaders,
traditional healers, female leaders) to engage and gain their support for leadership for CBS.
2. Selection: Select community informants or volunteers jointly with community leaders based on
certain criteria. Choose informants who possess a good character, who are invested with
community trust and acceptance, and who are knowledgeable of the area, live within the
community and speak the local language/dialect, as well as who represent an education level,
age and gender suited to the community culture and norms.
3. Support: Identify barriers and challenges that the community and/or informants may face,
particularly related to gender, and build support to resolve them. For example, evaluate an
informant’s access to information, literacy levels or training, decision-making power, or
restricted mobility/transport/money. Issues related to security and safety should also be
addressed, as well as the acceptability of tools, equipment and mobility, particularly for female
informants.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
66
4. Capacity building: Train community informants using concise educational materials, the
simplified AFP case definition, suspected AFP case recording and reporting policies, stool
collection and handling procedures, and clear roles and responsibilities. Provide materials to
support tasks, such as visual job aids, case investigation forms (CIFs), tools to record
information, focal point contact information, and stool collection kits.
5. Activities: Community informants/volunteers will:
• actively search for suspected AFP cases through rumours, regular (biweekly) home visits,
and more frequent (weekly) visits to traditional healers and religious leaders;
• keep records on vaccination and basic demographic data for families and children; and
• immediately report a suspected case of AFP to the designated CBS focal point and/or the
surveillance officer. The surveillance officer will follow up to confirm that the suspect AFP
case meets the AFP case definition, initiate investigation and specimen collection, and
notify the district health authority.
6. Supportive supervision: Establish an oversight structure that supports community
informants/volunteers by conducting regular supervisory visits and providing feedback and
conduct periodic refresher trainings to ensure informants maintain their knowledge and skills.
Challenges and troubleshooting
Certain challenges should be anticipated in setting up, implementing and maintaining CBS (Table 6.1).
Table 6.1. Issues and possible actions to troubleshoot community-based surveillance
Issue
Possible actions
Difficulty to sustain CBS
due to cost
● Build on existing local CBS networks.
● Explore less resource-intensive CBS modalities to balance available funds
with sufficient activities to address surveillance gaps.
● Advocate for internal resources and reinforce community and government
ownership of CBS (government budgets, bilateral cooperation) to ensure
continuity, rather than external support which may not be sustainable.
● Consider integrated surveillance (e.g., VPDs) or integrated interventions (e.g.,
health education and immunization) to share costs.
Difficulties finding the “right”
community volunteers, as many
programmes compete for
suitable volunteers and may
have different incentives
● Adapt case definitions, forms, protocols and training to the literacy level of the
community volunteers to carry out on-the-job mentoring and motivation.
● Coordinate and collaborate with other agencies and community networks and
use shared volunteers.
Difficulty in recruiting women as
community informants (due to
existing gender norms and rules
restricting women’s
participation, safety and security
risks faced by female frontline
workers, lower literacy rates,
women’s restricted mobility or
lack of acceptable modes of
transport)
● Systematically analyse and address gender-related barriers to increase
women’s meaningful participation, safety and job satisfaction. Engage with
community/religious leaders to pave the way for women’s participation.
● Develop strategies to increase gender balance among volunteers, including
actions for revising selection criteria, retention, equal remuneration and
capacity building; address specific barriers affecting women’s participation in
training activities (such as transport options, the timing and location of
training).
● Ensuring that policies and training for the prevention of all forms of
harassment, sexual exploitation and abuse and other forms of gender-based
violence (GBV) are in place, actively communicated and implemented, sharing
information about existing confidential reporting mechanisms and
safeguarding policies for community volunteers.
CBS = community-based surveillance; GBV = gender-based violence; VPD = vaccine-preventable disease
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
67
Table 6.1 (continued)
Issue
Possible actions
Lack of community
cooperation and trust
● Build trust by engaging the community in the selection process for volunteers,
in the recognition and motivation of volunteers, and in the provision of
feedback – all with respect to local social/cultural norms.
● Engage key influencers within communities, including women’s groups,
community organizations, religious leaders and other opinion influencers
(based on context analysis).
● Ensure the provision of observable benefits to the community (e.g.,
interventions, health education).
Ineffective communication
with targeted communities
● Consider including popular local media (radio, mobile messaging) to respond
to the different preferences, needs and challenges of diverse women and
men in the community (for example, different channels and platforms,
different literacy levels).
● Target both men and women as caregivers in all polio and AFP-related
community outreach, encouraging men’s increased participation in children’s
health care.
● Utilize toll-free numbers or communication networks to report AFP cases.
Difficulties in quickly conducting
AFP case investigation in
inaccessible areas and among
some special populations.
● Consider having the interview of the suspected AFP case (or collection and
transport of specimen) done by the community volunteer; ensure appropriate
training and coaching.
● Consider investigating the AFP case outside of his/her catchment area by the
community volunteer; ensure provision for transportation cost for examination
and/or specimen collection.
Limited ability or inability to
perform monitoring and
supportive supervision in
inaccessible or hard-to-reach
areas.
● Explore innovative ways of working remotely (e.g., phones, WhatsApp) or
relying on local organizations. Refer to Guidelines on Implementing
Poliovirus Surveillance in Hard-to-Reach Areas & Populations.
● Ensure means of communication for community volunteers and surveillance
officers: petty cash, phone or other access to means of communication.
● Consider using an electronic system for connecting informants’ activities and
suspected AFP cases to the public health system.
Waning interest and motivation of
informants over time which leads
to deteriorating reporting quality
and high turnover of staff
● Keep informants motivated. An integrated CBS may be more rewarding as
community informants can directly observe the benefits from their work.
● Provide a strong supervisory structure and regular feedback and periodic
refresher trainings.
● Maintain support and offer recognition for activities that are well done .
● Welcome the report of suspected AFP cases, even if they do not meet the
“true” AFP case definition.
Simplified AFP case definitions
make CBS less specific
● Balance the sensitivity and specificity of the overall CBS system with
repeated training, close supervision and feedback.
Increased workload in polio
laboratory
● Coordinate on a regular basis with the laboratory and inform them if expected
workload is likely to increase.
AFP = acute flaccid paralysis; CBS = community-based surveillance
Monitoring and evaluation
CBS should be well monitored and reviewed to guide timely corrective action (Table 6.2). Monitoring
activities can be done with the help of existing partners and community networks (e.g., community
mobilizers) and through engagement of local government authorities.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
68
Table 6.2. Indicators for community-based surveillance
Indicator
Calculation (expressed as a percentage)
Target
Proportion of AFP cases
reported by CBS
# of AFP cases (those on linelist) identified by community
informant
/
# of AFP cases on linelist
TBD
Completeness of
weekly/monthly zero reporting
(WZR/MZR)
# of reports received from community informants
/
# of expected reports from community informants
>=80%
Timeliness of WZR/MZR
# of reports received on time from community informants
/
# of expected reports from community informants
>=80%
Proportion of female
informants
# female informants
/
# informants
>=50%-80%*
Proportion of informants from
local area
# local informants
/
# informants
>=80%*
Supervision of informants
† ‡
# informants who have received at least one supervisory
visit in last 3 months
/
# number of informants
>=80%
Informant training
‡ §
# informants with training within the last year
/
# of informants
>=80%
Informant turnover rate
‡ § ¶
# informants who left during the previous year
/
# informants
TBD
AFP = acute flaccid paralysis; CBS = community-based surveillance; MZR = monthly zero reporting; TBD = to be determined;
WZR = weekly zero reporting
*Target to be adjusted at the country level; priority countries to regularly analyse.
†
To be reviewed quarterly; priority countries to regularly analyse. Suggest to stratify results by supervisor.
‡
Results should be stratified by sex.
§
To be reviewed annually; priority countries to regularly analyse.
¶
Informant turnover rate is a flag; the target is to be defined at the country level. The baseline is the number of informants at the beginning
of the review period.
The first three indicators can be monitored monthly, with the rest monitored annually.
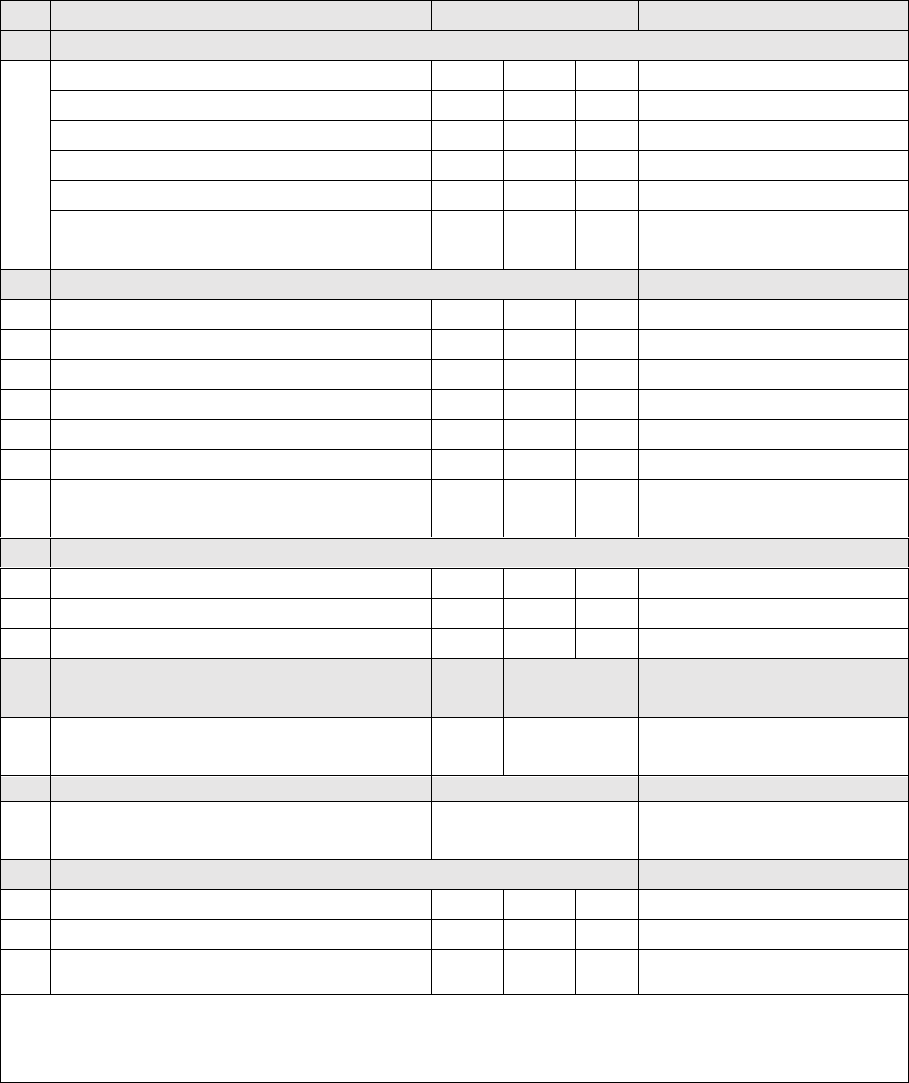
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
69
Annex 7. Examples of forms
7.1 - Active surveillance visit form
Active surveillance (AS) for acute flaccid paralysis (AFP)
AS visit report form
Name of officer: ____________________
Date of visit: ___________________________
Year
___________________________
Month of visit: __________________________
Province: __________________________
District: _______________________________
Name of health facility (+ other identifier): ______________________________________________________________
No.
Item
Status
Remarks
1
Interview with:
1.1
Doctor in charge
Yes
No
N/A
1.2
AFP / surveillance focal point
Yes
No
N/A
1.3
Paediatrician of the facility
Yes
No
N/A
1.4
Neurologist of the facility
Yes
No
N/A
1.5
Physiotherapist of the facility
Yes
No
N/A
1.6
Other health facility staff. Specify:
___________________________________
Yes
No
N/A
2
Check for new / missed AFP cases:
Details of new AFP cases:
2.1
Outpatient register (OPD) checked for AFP cases
Yes
No
N/A
2.2
Inpatient register (IPD) checked for AFP cases
Yes
No
N/A
2.3
Internal medicine department / ward
Yes
No
N/A
2.4
Neurology unit
Yes
No
N/A
2.5
Orthopaedic department
Yes
No
N/A
2.6
Physiotherapy unit
Yes
No
N/A
2.7
Other departments / units / wards. Specify:
___________________________________
Yes
No
N/A
3
Check for supplies and material availability:
3.1
Stool specimen kit(s)
Yes
No
N/A
3.2
Specimen carrier(s)
Yes
No
N/A
3.3
AFP poster(s) visible in the facility
Yes
No
N/A
4
Summary:
New and unreported cases since last visit:
New
(all new)
Unreported
(out of the new
cases found)
If already reported, write EPID no.
4.1
Number of AFP cases found during this visit, since
the last visit
5
Feedback:
Number
EPID of cases for result pending
5.1
Number of AFP cases for which results have not
reached the facility in >60 days
6
Other checks done:
Remarks
6.1
Vaccine cold chain fully functional
Yes
No
N/A
6.2
Polio vaccine in stock
Yes
No
N/A
6.3
Other: __________________________________
Yes
No
N/A
Name of person in charge of facility: __________________________
Signature of person in charge of facility: _______________________
Date: ___________________
Signature of officer: ___________________________________
Date: ___________________

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
70
7.2 - Case investigation forms (version 2022 for non-endemic and endemic)

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
71
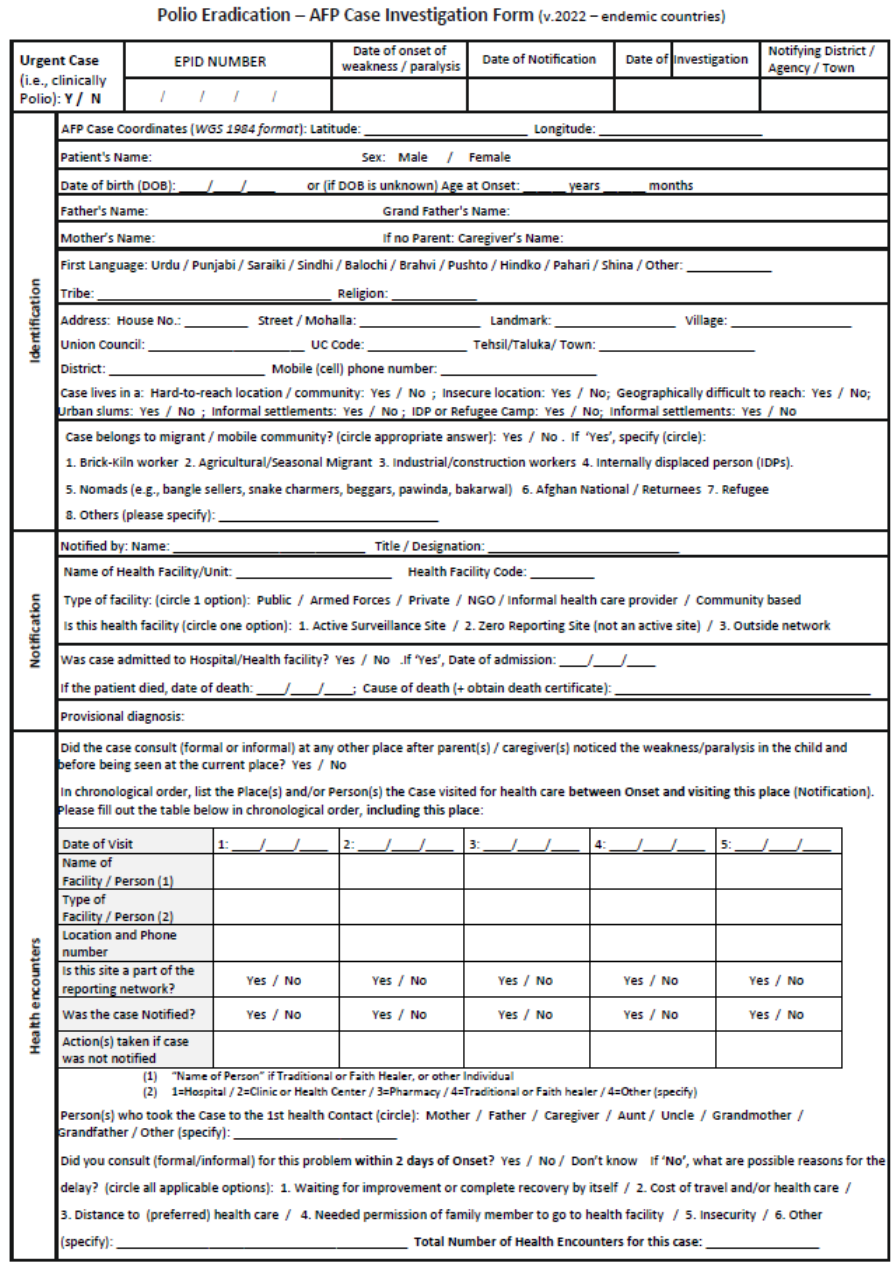
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
72

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
73
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
74
7.3 - Detailed case investigation form
The main elements to include in a detailed case investigation form (CIF) or report are:
29
● Case notification
- Name and unique epidemiological identification
(EPID) number
- Date of notification
- Name of respondent and relationship with case
- Name of interviewer, contact information and
affiliation
- Date of case investigation
● Demographic
- Residence (province, district, village, etc.)
- Date of birth, age
- Sex
● Vaccination
- Total number of oral polio vaccine (OPV) doses
received in essential immunization (incl. code for
unknown, i.e., 99)
- Total number of OPV doses received during
supplemental immunization activities (SIAs) (incl.
code for unknown, i.e., 99)
- Total number of inactivated polio vaccine (IPV)
doses received in essential immunization (incl.
code for unknown, i.e., 99)
- Total number of IPV doses received in SIAs (incl.
code for unknown, i.e., 99)
- Date of last OPV dose
● Clinical information
- Date of paralysis onset
- Fever at onset of paralysis?
- Asymmetric paralysis?
- Neurological examination
● Risk factors
- Occupation of parents/caregivers
- Ethnicity
- Special population (check all that apply): refugee,
internally displaced population (IDP), reside in
security-challenged area, migrant/mobile population
- Travel history of case and household members
(outside of district or country) within one (1) month of
onset of paralysis
- History of attendance at gathering of case and
household members (large scale market/fair, other)
within one (1) month of onset of paralysis
- History of visitors to the household within one (1)
month of onset of paralysis
● Specimens
- Specimen numbers
- Date of collection of stool specimens
- Date stool specimen received in laboratory
- Condition of stool (good, poor, unknown)
● Laboratory results
● History of care-seeking prior to notification
- Name and location of sites / facilities visited by the
case between onset and notification
- Dates of visits
● Other AFP cases in area?
● Geographic and demographic information,
population size of area
● Rapid OPV/IPV coverage survey of area
● Essential immunization and SIA coverage
● Map
If the polio isolate was detected through environmental surveillance (ES), special focus should go
towards understanding the catchment area of this ES site, the sociodemographic characteristics and
level of vaccination coverage of the population living in that catchment area. In addition, the
investigation should look for missed AFP cases in/around the ES site catchment area.
29
An example of a detailed case investigation form can be found on the GPEI website (http://polioeradication.org/wp-
content/uploads/2016/09/Detailed-Case-Investigation-Form_July2011_EN.doc).

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
75
7.4 - 60-day follow-up examination form

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
76

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
77
Annex 8. AFP case investigation
How to document the case history
While observing the patient for signs of paralysis or weakness, the surveillance officer should take the
history of the case from the patient’s caregiver (or the patient, if an older child), transcribing key
elements on the case investigation form (CIF), including:
(1) Patient identification
• Patient / caregiver identification (names, address or location, mobile phone, etc.) that will be
key to tracing the family back, if needed.
• Date of onset of paralysis. Key for further analyses.
(2) Immunization history
• Number of oral polio vaccine (OPV) and/or inactivated polio vaccine (IPV) doses received prior
to onset of weakness, whether through supplementary immunization activities (SIA) or essential
immunization (confirm with immunization card, if available).
• Siblings (OPV and/or IPV) vaccination status.
(3) History of illness
• First symptoms; date and place of onset of weakness or paralysis (key for the assignment of
the epidemiological identification [EPID] number); fever or other symptoms at onset, incl.
whether the weakness progressed rapidly or not, and whether the weakness affected both
extremities equally or not.
• If one or more health providers (formal, informal) were consulted prior to the case being
notified, this should be noted, as well as the dates and the names of providers and what
treatment, if any, was provided.
• The caregiver should be asked whether there is anyone else in the community with similar
symptoms.
(4) Travel history
• Travel by the case or anyone else in the household during the 30 days prior to onset of
weakness (record details: person, place, time).
• Visitors received during the 30 days prior to onset of weakness (record details: person, place,
time).
(5) Special population or high-risk group
• Nomads, internally displaced population (IDP), refugees, people living in inaccessible areas, or
other special population or high-risk group should be recorded on the CIF, if applicable.
How to conduct the examination
The objective of the clinical examination in a case
investigation of acute flaccid paralysis (AFP) is to
establish whether there is any degree of paralysis or
paresis or not, regardless of the current clinical
diagnosis. It is therefore NOT to establish an exact
medical-neurological diagnosis. The physical
examination should then be done ideally by a person
qualified to do so – either the person charged with the investigation or the attending physician in the
hospital.
In most cases, the investigator will have learned much about the presence or absence of flaccid
paralysis just through the initial observation of the patient. Depending on the patient's age and ability to
cooperate, the investigator should request the patient to walk (if there is an involvement of lower limbs)
In AFP surveillance, the objective of the
clinical examination is to establish whether
there is any paralysis or paresis or not. It is
NOT to establish an exact medical-
neurological diagnosis.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
78
and then observe the patient's gait. If there is involvement of the upper limbs, request the patient to lift
his/her arms. While the physical examination is easier with a cooperative older child, it must also be
done with infants and toddlers, and thus, trust must be secured.
The focus of the examination should be on simple neurological testing, including an assessment of
motor power, muscle tone and reflexes. Status of sensation should be verified. A brief overall clinical
examination should be conducted to assess the health status of the child, including a temperature
check for a fever and any signs of malnutrition and dehydration. Where / when feasible, a neurological
examination through a paediatrician or neurologist can be carried out and attached to the CIF but is not
essential.
How to collect and store stool samples for AFP cases
Materials and supplies
✓ Specimen carrier
✓ Frozen ice packs (4)
✓ Case investigation form (CIF)
✓ Laboratory request form
✓ 2 screw-top specimen
collection containers
✓ Container labels (adhesive)
✓ Water-resistant pen
for labelling
✓ Absorbent material
(e.g., cotton)
✓ Gloves
✓ 4 Ziploc plastic bags (to hold
containers and forms)
✓ Contact information
of parent/guardian
✓ EPID numbers,
if available
Step-by-step instructions
For a process flow on collecting stool samples for AFP
cases, see Fig. 8.1.
1. Use only the designated stool carrier (not the
carrier used for vaccines), which should be lined
with frozen ice packs.
2. Use the designated screw-top specimen
containers. Should such containers not be
available, use any dry, clean, leak-proof
container or bottle.
3. WEAR GLOVES DURING SPECIMEN
COLLECTION!
4. Collect fresh stool from the patient’s diapers or
bed pan, or have the patient defecate onto a
piece of paper or plastic.
5. Collect a volume of stool about the size of two
adult thumbnails (approximately 8-10 grams).
Note that the laboratory may reject extremely
watery samples and the laboratory also
considers rectal swabs inadequate.
6. Use the spatula provided in the kit container to
place the specimen in a clean, leak-proof, screw-capped container and firmly screw the cap
back on.
7. Use an indelible or permanent marker to record the following on the self-adhesive label (or a
piece of tape or directly on the container, if labels are not available):
o First and last name of the case
o EPID number
o Date of collection for each specimen
o Time of collection for each specimen
o Specimen number (“1
st
” or “2
nd
”)
For patients who need more time to
produce a specimen, leave all materials
listed above in the health facility or with
the family. Explain the collection
procedure in simple language. Return to
collect the specimens and provide new
frozen ice packs.
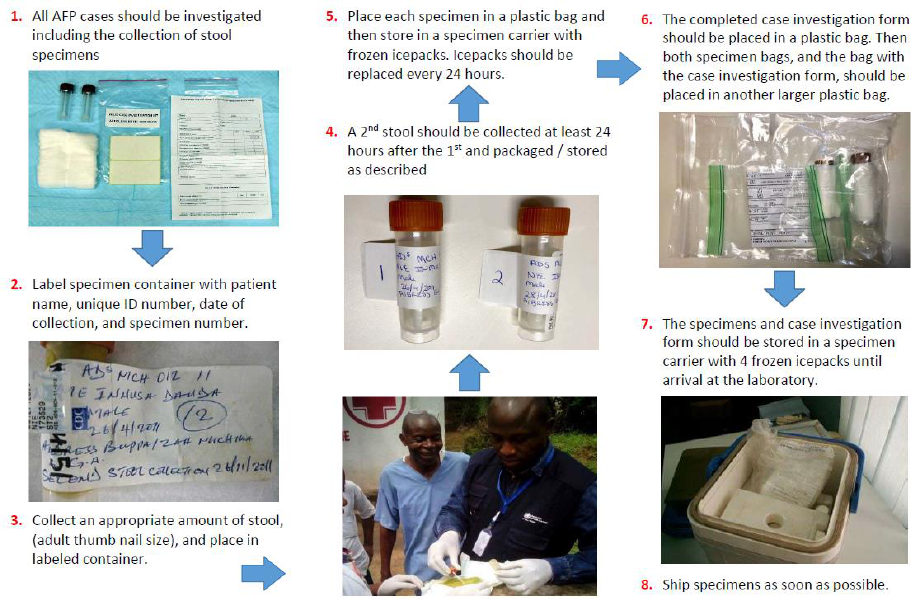
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
79
o “Hot case”
8. Stick the label to the appropriate specimen container.
9. Firmly close the container, place it in the Ziploc plastic bag, and seal the bag. If available, wrap
the container in absorbent material prior to placing in the bag in case of shock or leak during
transport.
10. Immediately place the specimen into the specimen carrier, in the middle of the four (4) frozen
ice packs. Never store stool samples in refrigerators or freezers with vaccines or food.
11. Remove gloves and dispose of them appropriately. Wash hands with soap and water after the
completion of specimen collection and glove disposal.
12. Repeat steps 1-11 for the second sample, to be collected at least 24 hours after the collection
of the first specimen.
13. Replace ice packs with new, frozen ice packs every 24 hours.
14. Once both stool samples are in the carrier, pack the remaining empty space in the carrier with
paper or cotton so that the containers do not move when the carrier is transported.
15. Place the completed CIF in a Ziploc plastic bag and place it in the carrier.
16. Place the completed laboratory request form for the case in a sealed Ziploc plastic bag and
place inside the carrier before sending to the laboratory.
(Fig. 8.2 offers further illustration on how to pack a specimen carrier.)
Fig. 8.1. Process flow for collecting stool samples for AFP cases
Source: WHO.
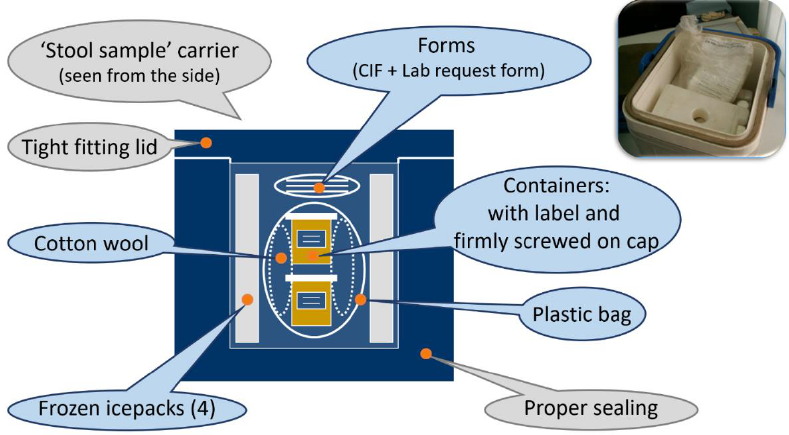
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
80
Fig. 8.2. Side view of a stool specimen carrier with the placement of material and supplies.
Source: WHO.
Additional storage information
Store specimens according to when they can be sent to the laboratory:
• ≤72 hours after collection, store in specimen carriers with frozen ice packs.
• >72 hours after collection, store in a deep freezer (-20°C) until transport. Do not freeze with
vaccines or food.

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
81
Annex 9. Health-seeking behaviour
Delays in detecting cases or missing cases altogether may arise from a limited understanding of the
health-seeking behaviour of acute flaccid paralysis (AFP) cases and their caregivers, as well as the
barriers they may experience in accessing health care.
To address this, country programmes must collect health-seeking behaviour data disaggregated at the
lowest possible administrative level by gender and by risk status, for example in the case of special
population groups. When analysed, such data can point to possible subnational surveillance gaps and
may help strengthen programme activities through a deeper understanding of the underlying causes
Case investigation forms (CIFs) should be modified to
include the following:
• the number of health encounters the case had
before it was notified;
• whether the reporting sites (facility/person) that
saw the case before it was notified are part of the
reporting network; and
• whether or not the encounter(s) led to a
notification.
Fundamentals of health-seeking behaviour assessments
• Why: Health-seeking behaviour assessments aim to identify healthcare facilities or persons that
cases and their caregivers seek out, but that may miss reporting AFP cases or may report
cases but are not currently in the AFP reporting network.
• What: Once these individuals or facilities have been identified, the programme can take the
appropriate action to increase the sensitivity of the AFP surveillance system – for example, by
re-training a focal point on AFP reporting or by adding a focal point to the reporting network.
• When: Health-seeking behaviour assessments can be coordinated as part of the periodic
review of the reporting network or during outbreak response assessments (OBRAs),
surveillance reviews or other activities aimed at reviewing and strengthening AFP surveillance.
• How: These assessments review information collected on modified CIFs where AFP cases
detail their health encounters before their case was officially reported through the AFP active
surveillance network.
Steps of a health-seeking behaviour assessment
1. Review the reporting network through analysis of CIFs to answer the following questions:
o How many reporting sites missed reporting an AFP case? Which ones, and where?
o What are the sites outside the network (i.e., not part of the reporting network) that (a)
received and (b) reported an AFP case?
2. Review for possible clusters of AFP cases that were detected late with the aim to identify
geographical areas where delays in detecting AFP cases may be linked to particular habits or
attitudes within a special population towards health care and seeking care, or where AFP
surveillance may be overlooking local, more traditional service providers.
3. Identify and implement actions to close surveillance gaps based on health-seeking behaviour of
a particular community (Table 9.1)
Countries should make sure that their
case investigation forms (CIFs) are
revised to collect data on previous health
encounters that cases had before they
were officially reported.
Refer to CIFs in Annex 7 for a section on
previous health encounters to capture
health-seeking behaviour information.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
82
Table 9.1. Specific actions to close surveillance gaps related to health-seeking behaviour
Situation
Action
Case went to a
reporting site but was
not notified
● Identify the possible reason(s) a case was missed (e.g., staff turnover, untrained
recruit, vacation, workload, case absconded) and address the gap.
● Review the prioritization (i.e., high-, medium-, low-priority sites); monitor and supervise
closely for 6 months for any missed cases
Cases seek care in a
health facility or site
that is not included in
the network
Conduct a visit to each health facility/site/person (if feasible) and evaluate the need for
inclusion in the reporting network:
● If the location/person fits the criteria (high-, medium-, or low-priority site), include it in
the network and sensitize on the need to report immediately.
● If the location/person does not meet the criteria, sensitize and monitor for 6 months for
additional cases. If additional cases, reconsider inclusion as reporting unit.
Clustering of late
detected cases
(cases that were not
reported upon their 1st
visit or were notified
beyond 7 days after
onset of paralysis)
● Conduct quick social mapping of area to identify possible reasons (e.g., high-risk
group, limited coverage of health facilities).
● If feasible, visit the area.
● Discuss with community the possible reasons for delays.
● Sensitize communities.
● Sensitize and train healthcare providers.
● Consider introducing CBS (after need assessment as per Annex 6).
Health-seeking behaviour should be monitored to guide timely corrective action (Table 9.2).
Table 9.2. Health-seeking behaviours indicators
Indicator
Calculation (expressed as a percentage)
Target
AFP case encounters
‡
# of AFP cases with <=2 health encounters
between onset and notification
/
# of AFP cases
>=80%
Adequacy of notification
by designation
# of 1st health encounters that led to a notification,
by designation [reporting source]
§
/
# of health encounters by that same designation
>=80%
Appropriateness of
surveillance network
# of AFP cases with first health encounters with a reporting
site within the AFP surveillance network
/
# of AFP cases
>=80%
Late reported AFP cases:
Completeness of health
encounter information
Among AFP cases reported >14 days after paralysis onset:
# of AFP cases with no information on health encounters
/
# AFP cases reported >14 days after paralysis onset
>=80%
§
This is the “percentage of 1
st
encounters by designation (e.g., doctor, nurse, traditional healer, vaccinator, other) that led to the
notification of an AFP case.”
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
83
Annex 10. Special population groups
Special population groups
Definition
Special populations are groups that are not served or are underserved by the regular health
delivery system.
Categories
1. Populations living in security-compromised areas
2. Mobile populations: nomads and seasonal migrants (e.g., agricultural or mine workers,
brick kilns, construction workers, etc.)
3. (a) Refugees and IDPs in camps and (b) those living in host communities
4. Special populations in settled areas (e.g., cross-border population, urban slums,
islanders, fishermen, etc.)
Identification &
mapping
It is important to identify and profile these populations based on:
● geographic location, population size, movement routes, timing/seasonality of movement;
● access to health services, health-seeking behaviours, ability of the current surveillance
network (health facilities, community-based) to detect AFP cases within the group;
● identification of service providers (public and private, including NGO’s, faith-based
organizations, etc.);
● vaccination coverage and immunity status; and
● availability of communication activities targeting these special population.
Rationale for
special activities
to reach
particular
populations
These populations may have more susceptibility to the disease and more likelihood of missing
and spreading transmission.
● Underserved populations may not be covered by the surveillance system.
● There is likely lower population immunity due to low vaccination.
● High movement makes them prone to spread the virus to vulnerable populations.
Challenges and
anticipated issues
for surveillance
among special
populations
● Difficulties with mapping and population estimates
● Lack of coordination with stakeholders
● Lack of community involvement
● High cost of resources and logistics: trainings, transportation, supervision, monitoring
● Lack of security
Tips for success
Special population surveillance is facilitated by:
● Special teams dedicated to surveillance in special population
● Close coordination with partners (UNHCR, IOM, INGOs, civil society, veterinary
services, etc.)
Surveillance
strategies
applicable to the
special
population
1. Populations living in security-compromised areas
● Access mapping and analysis that identifies key partners and factions, population
dynamics and changes.
● Access negotiating
● Sensitizing and briefing armed forces, relevant partners and community members about
polio and AFP case reporting.
● Revising surveillance network by identifying and training appropriate focal points for
case reporting— i.e., community-based surveillance (CBS) as appropriate.
● Conducting periodic active case search in community and healthcare facilities.
● Contact sampling around AFP cases (one sample, three contacts).
● Conducting healthy children stool surveys and ad hoc environmental surveillance (ES),
to be decided in coordination with WHO country and regional teams.
● Ensuring access tracking and segregated data analysis to monitor surveillance by
population group.
AFP = acute flaccid paralysis; CBS = community-based surveillance; ES = environmental surveillance; IDP = internally displaced
population; INGO = International nongovernmental organization; IOM = International Organization on Migration; NGO = nongovernmental
organization; UNHCR = United Nations High Commissioner for Refugees; WHO = World Health Organization

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
84
Special population groups (continued)
Surveillance
strategies
applicable to the
special
population
2. Mobile populations
● Mapping and profiling with leaders or persons identified as surveillance focal points.
● Determining itineraries of the population and mapping healthcare facilities and providers
(including veterinarians) along the route.
● Sensitizing population and providers.
● Conducting market sensitization along the route and close to water points and camps.
● Establishing regular contact with focal points for reminders and feedback on reporting.
● Conducting active case search in large gatherings of nomadic groups during SIAs and
mobile outreach services.
● Collecting contact sampling around AFP cases (one sample, three contacts).
● Conducting healthy children stool surveys to be decided in coordination with WHO country
and regional teams.
A similar approach will be used for other mobile population groups as appropriate – e.g., seasonal
migrants such as agricultural or mine workers, brick kilns, or construction workers.
3a. Refugees/IDPs in camps
● Identifying focal points in camps (IDP or refugee) to include in the surveillance network.
● Profiling new arrivals (origin and immunization status).
● Conducting active case search in health facilities of camps and during SIAs.
● Collecting contact sampling around AFP cases (one sample, three contacts).
● Collecting healthy children sampling (new children under five year), to be decided in
coordination with WHO country and regional teams.
● Installing a permanent vaccination/surveillance team.
3b. Informal IDPs and refugees in host community
● Identifying key informants from the community to include in surveillance network.
● Providing appropriate job aids.
● Initiating community IDP and refugee tracking (tracker team).
● Determining health-seeking behaviour.
● Adjusting surveillance network.
● Conducting active case search during SIAs and mobile activities.
● Collecting contact sampling around AFP cases (one sample, three contacts)
● Collecting healthy children sampling (health facilities used by IDPs or refugees), to be
decided in coordination with WHO country and regional teams.
4. Special populations in settled areas
Cross-border populations
● Mapping official and non-official border crossings
● Mapping seasonal movements
● Estimating population flow averages
● Mapping and profiling villages/settlements, special populations, security and access,
gathering places on both sides
● Mapping areas of one district/country only accessible from the neighbouring district or
country
● Mapping of surveillance network on both sides
● Identifying organizations working at border entry and exit points (e.g., immigration, port
health services, police)
● Providing orientation and sensitization of populations and healthcare providers on both sides
● Using supplemental strategies
● Active case search on both sides in the community (entry points, permanent vaccination
sites, markets) and in health facilities
● If there are security-compromised areas or special populations as refugees or IDPs,
implement the specific proposed activities/strategies
AFP = acute flaccid paralysis; IDP = internally displaced population; SIA = supplementary immunization activity; WHO = World Health
Organization

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
85
Special population groups (continued)
4. Special populations in settled areas (continued)
Urban slums
● Profiling communities and their origin
● Studying health-seeking behavior and modification of surveillance network
● Conducting active case search
● Consider adding ES sites
Monitoring and
Evaluation
● Conduct a segregated analysis to ensure surveillance coverage and quality by population
groups (starting with appropriate data collection)
● Conduct regular mapping and risk assessment
● Review/assess implementation of plans
● Engagement of partners for independent monitoring
ES = environmental surveillance

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
86
Annex 11. Ad hoc active case search
Ad hoc active case search for AFP cases
Definition
Ad hoc active case search (ACS) is an extraordinary, ad hoc activity conducted to identify
unreported acute flaccid paralysis (AFP) cases.
ACS is done through retrospective case search in health facility records and interviews of
healthcare providers (facility-based) and community leaders and parents (community-based). As
an ad hoc activity, ACS enhances active surveillance (AS) activities in the short term under
certain criteria, such as a new event or outbreak or when other concerning surveillance gaps are
identified.
Rationale and
indications
ACS is done to enhance the sensitivity of detecting AFP cases in areas that experience either
suboptimal surveillance or new epidemiological risks. This activity can help identify gaps in the
AFP surveillance system when new events or outbreaks occur – and it can help supplement
activities during the beginning of a response plan.
Conditions that may warrant ACS include:
1. Activities where opportunities to look for AFP cases exist, such as during house-to-house
searches, while canvassing to collect geospatial data, while vaccinating newly accessible
populations (e.g., refugees or internally displaced populations [IDPs] from inaccessible
areas), or during supplementary immunization activities (SIAs).
2. Events, outbreaks and other triggers
a) In a polio event or outbreak setting
i) As part of the investigation, retrospective case searches and facility-based ACS are
implemented.
ii) As part of enhanced surveillance by activating AFP case finding and record review
b) Other trigger indications
i) A disconnect between environmental surveillance (ES) and AFP surveillance
findings (i.e., when wild poliovirus (WPV) or vaccine-derived poliovirus (VDPV) is
detected in ES and not through AFP).
ii) Clustering of polio-compatible cases in time and space.
While AFP surveillance implementation or enhancements are being made, ACS can fill a
surveillance gap in the short term:
1. Sizable population arrival and settlement, such as IDPs, refugees, and nomads coming
from high-risk areas with a recent outbreak or polio event
2. New access to previously inaccessible areas
3. Silent districts or areas
4. Low-performing surveillance areas*
5. When surveillance reviews identify gaps in surveillance performance
* While facility-based case search may be recommended in such instances, community-based case search is not
recommended unless warranted by further review.
Procedure
(steps)
Setting up ACS can be resource-intensive, so it’s important to have clear parameters, including
the geographic scope, target population and time period of interest (typically previous 6 months).
Geographic scope can be defined in review of outbreak-related risk assessments, current
epidemiology, genetics of new polio cases or other important risk factors to identify unreported
cases. When there are positive ES samples but no AFP case, the geographic scope may be more
complex because of the catchment area, requiring additional planning considerations.
ACS involves all or a subset of activities, depending on the situation. The steps below can be
considered in setting up ACS activities, but it is important to be focused so the search doesn’t
become larger and more resource-intensive than needed. Activities should be consistently
documented throughout the entire process.
ACS = active case search; AFP = acute flaccid paralysis; AS = active surveillance; ES = environmental surveillance; IDP = internally
displaced people; SIA = supplementary immunization activity; WPV = wild poliovirus; VDPV = vaccine-derived poliovirus
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
87
Ad hoc active case search for AFP cases (continued)
Procedure
(steps) -
continued
1. Conduct an analysis of AFP surveillance indicators.
2. Conduct subgroup analysis to determine if surveillance reaches all subsets of a population.
3. Decide if the search will be facility- and/or community-based (usually both).
4. Develop tools (e.g., checklist, reporting formats) for recording ACS process and outcomes.
5. Consider enlisting help from nongovernmental organizations (NGOs) for inaccessible areas.
6. Provide training to those who will conduct searches.
7. Develop reporting channels for identified AFP cases.
8. Establish a strong supportive supervision and monitoring mechanism at the field level.
Additional steps for facility-based ACS
1. Identify and profile all healthcare facilities within and outside the reporting network (public,
private, traditional).
2. Retrospective case searches should look for unreported AFP cases up to 6 months prior to
the search date. (Interview health providers, review facility registers, make visits to wards.)
Additional steps for community-based ACS
1. Map and profile areas and populations and identify leaders or contact persons.
2. Ensure community engagement for information gathering and facilitation (e.g.,
IDPs/refugees: identify elders, camp management committee, host community informants).
3. House-to-house case search, community case search.
All AFP cases should be added to the line list and should follow case investigation guidelines,
including stool specimen collection within 60 days of paralysis onset and contact sampling.
Frequency
This is generally an ad hoc activity when new events/outbreaks are identified in initial response.
Other situations where this activity could be considered, if resources allow: when a window of
opportunity opens in fully or partially inaccessible areas. In recently accessible areas with
disrupted healthcare infrastructure, the frequency should be every 3–6 months.
Challenges
and
anticipated
issues
ACS has challenges such as:
● Lack of resources: untrained personnel, poor documentation, or inadequate budget.
● Security issues.
● Lack of access to, poor quality or non-availability of health facility records.
● Logistical constraints in reaching communities and health facilities.
Enabling
factors & tips
for success
ACS is facilitated by:
● Community engagement.
● Presence of NGOs in inaccessible areas.
● Careful, in-depth analysis to prioritize areas, populations or health facilities.
● Knowledgeable and motivated field staff, experienced supervisors.
● Good ACS documentation.
Interpretation
of results
● The detection of unreported AFP cases demonstrates gaps in the AFP reporting network.
● Retrospective review of records in facilities within the reporting network will reflect
whether regular active surveillance of designated sites was conducted.
Interviewing traditional healthcare providers and/or private sector practitioners will reflect whether
the local surveillance team has been orienting and contacting them. It may also highlight the need
to revise the reporting network.
Monitoring &
evaluation
● Number of unreported AFP cases detected through ACS (1) with onset less than 60 days
and (2) with onset more than 60 days to six months (or older).
● Number of communities and health facilities that had unreported AFP cases found in the
process.
● Assess impact of this activity on overall surveillance system, document any changes in
routine active surveillance or reporting networks, and develop and implement
improvement plans, where needed.
ACS = active case search; AFP = acute flaccid paralysis; NGO = nongovernmental organization

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
88
Annex 12. AFP contact sampling
AFP contact sampling
Also known as
Direct contact sampling and close contact sampling
Definition
The collection and testing of one (1) stool specimen from three (3) individuals in contact with
an acute flaccid paralysis (AFP) case. Children in frequent contact with an AFP case (e.g.,
touching, sharing toys, and sharing food) should be identified for specimen collection.
Surveillance guidelines recommend:
● Children, preferably <5 years of age.
● In contact with AFP case within a week prior to and/or two weeks after paralysis onset.
● Examples include siblings and other children living in the same household and/or
neighbouring children who played with the AFP case during the period of interest.
● Stool specimens from AFP case contacts may be collected up to 60 days after
paralysis onset, as poliovirus may be excreted up to two (2) months or longer.
● Stool specimens are typically collected from the community of residence of the AFP
case. However, if the AFP case stayed in other communities one week prior to and/or
two weeks after paralysis onset, then collection of specimens from contacts of the AFP
case at these locations may also be warranted.
Purpose and
rationale
AFP contact sampling is used to provide laboratory evidence of poliovirus in an AFP case.
Individuals in contact with AFP cases have a higher likelihood of asymptomatic infection and
virus excretion than people who have not had contact. The collection of stool specimens from
contacts of AFP cases provides an additional approach to determine if poliovirus is the cause
of paralysis in an AFP case. Positive laboratory results of contact specimens are used to
confirm poliovirus infection in an AFP case who is not otherwise laboratory-confirmed.
Indications
AFP contact sampling should be performed as part of regular AFP surveillance activities.
Expanded use of AFP contact sampling may also be done as part of outbreak response
activities.
● Regular AFP surveillance activities: Recommendations per the Global Polio
Surveillance Action Plan 2022–2024 for AFP contact sampling.
● All AFP cases with inadequate stool specimens. Examples of inadequate stool
specimens are: (a) 0 or 1 stool specimen collected; (b) at least one stool specimen
collected > 14 days after paralysis onset; (c) two stools collected <24 hours apart; and
(d) poor stool condition (e.g., specimen was hot upon arrival at laboratory).
● After close coordination with national surveillance and laboratory colleagues, consider
all AFP cases who reside in security-compromised or hard-to-reach areas to take
advantage of the limited opportunity to reach these individuals and communities.
● Outbreak response activities: Expansion of AFP contact sampling to enhance AFP
surveillance may be warranted under specific circumstances. Expansion should occur
in close coordination and collaboration between the national surveillance and
laboratory colleagues.
○ All AFP cases in an outbreak-affected country, to improve detection of all viruses
○ All AFP cases detected outside the subnational outbreak zone, to increase the
probability of detecting virus movement beyond the designated outbreak zone
IMPORTANT: Results from AFP contact sampling cannot be used to confirm community-wide transmission of
poliovirus. Because laboratory results cannot be used to guide surveillance or outbreak response activities,
collection of stool specimens is not recommended from contacts of individuals with following classifications: (1)
WPV, aVDPV, cVDPV, unclassified VDPV, SL2 positive; (2) poliovirus positive contacts of AFP cases; and/or (3)
poliovirus positive healthy children.
AFP = acute flaccid paralysis; aVDPV = ambiguous vaccine-derived poliovirus; cVDPV = circulating vaccine-derived poliovirus; SL2 =
Sabin-like type 2; WPV = wild polio vaccine
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
89
Additional important information
When to conduct
AFP contact sampling should be conducted during the initial or follow-up activity of an AFP
case investigation (i.e., before laboratory results are available).
● Initial AFP case investigation: Conduct AFP contact sampling if it is known that two
stool specimens cannot be collected in a timely manner.
● Follow-up activity: Conduct AFP contact sampling if the laboratory reports that the
AFP case’s stool specimens were received in poor condition.
Specimen labelling
Each specimen should be labelled clearly as a contact of the AFP case. The unique
identification number should be the same as the AFP case with an added contact indicator
(“C”) and number (#) suffix (e.g., C1, C2, C3).
“Other”
classification
Positive AFP contacts are not classified as confirmed poliovirus cases because they do not
meet the case definition, which requires acute flaccid paralysis. Results are included as
“others” in poliovirus isolation counts.
Procedures
Refer to the GPEI Global Polio Surveillance Action Plan 2022–2024 for further details.
AFP = acute flaccid paralysis; GPEI = Global Polio Eradication Initiative
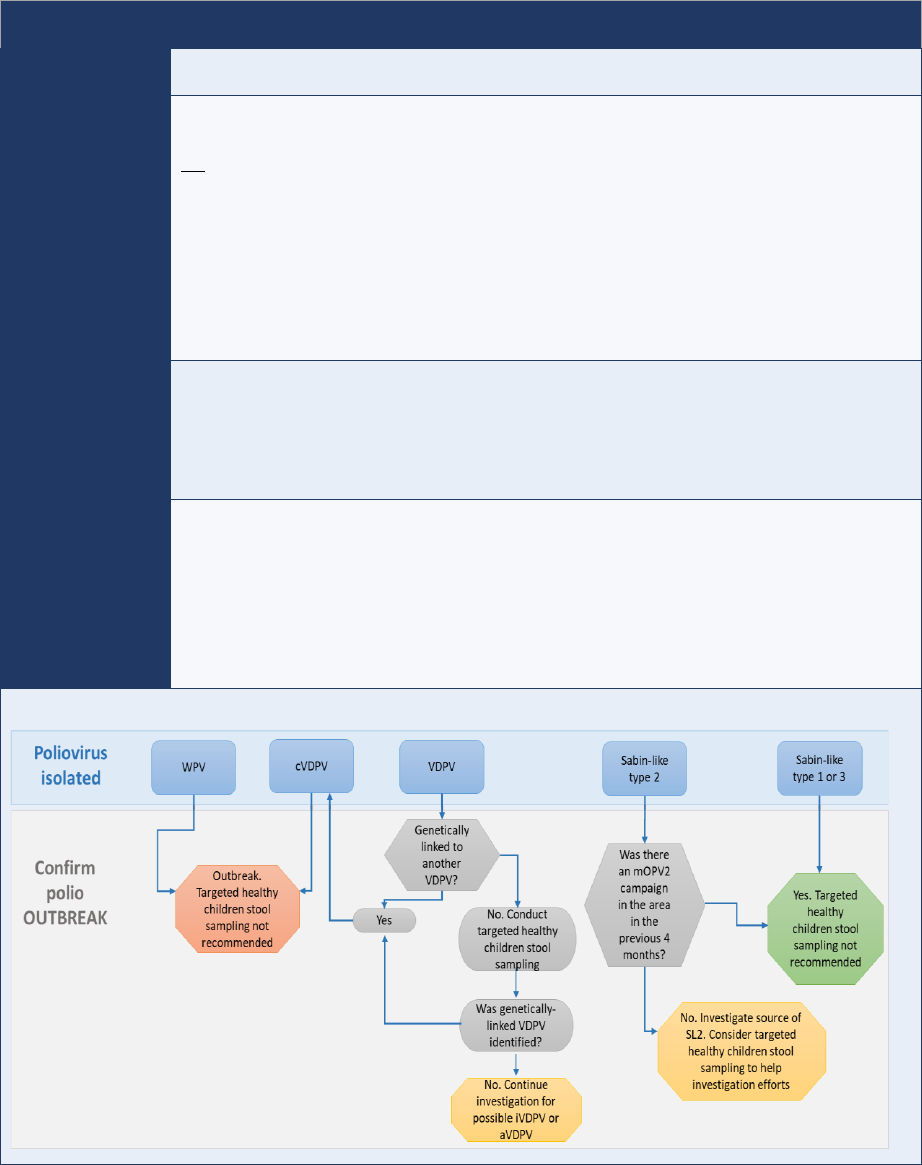
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
90
Annex 13. Targeted healthy children stool sampling
This job aid is available online (https://polioeradication.org/wp-content/uploads/2020/03/AFP-contact-
sampling-and-targeted-healthy-children-stool-sampling-20200327.pdf).
Targeted healthy children stool sampling
Also known as
Healthy children sampling, community stool sampling and community sampling
Definition
The collection and testing of one (1) stool specimen from 20 healthy children to determine if
there is community-wide transmission of poliovirus (i.e., outbreak). Healthy children who have
not had contact with the confirmed poliovirus case should be targeted for specimen collection.
Surveillance guidelines recommend:
● ideally children <2 years old, though can be up to 5 years old;
● not in contact with the confirmed poliovirus case within the week prior to or two weeks
after paralysis onset (i.e., not a contact);
● healthy with no evidence of acute flaccid paralysis (AFP); and
● specimens collected from the same community as the positive poliovirus case,
specifically in another part of the community and not an immediate neighbour.
Purpose and
rationale
Targeted healthy children stool sampling is conducted to determine if there is community-wide
transmission of poliovirus. Community-wide transmission indicates an outbreak, which requires
mobilization of resources to quickly launch an outbreak response. The collection of specimens
from healthy children who have NOT been in contact with the positive poliovirus case is critical to
establishing confirmation of community-wide transmission.
Indications
Targeted healthy children stool sampling is useful in a very limited number of situations during an
event or outbreak investigation, specifically those situations when community-wide transmission
has yet to be confirmed. In situations where an outbreak has been confirmed, the use of targeted
healthy children stool sampling is discouraged as it would be an inefficient and ineffective use of
programme resources. Any decision to do a targeted healthy children stool sampling should be
made in close coordination and collaboration with national surveillance and laboratory
colleagues.
Fig. 13.1. Flow chart for assessing situations for targeted healthy children stool sampling
AFP = acute flaccid paralysis; aVDPV = ambiguous vaccine-derived poliovirus; cVDPV = circulating vaccine-derived poliovirus; iVDPV =
immunodeficiency-associated vaccine-derived poliovirus; SL2 = Sabin-like type 2; mOPV2 = monovalent oral polio vaccine type 2; VDPV
= vaccine-derived poliovirus; WPV = wild poliovirus

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
91
Additional information
Notes on
indications for
targeted healthy
children stool
sampling
(see Fig. 13.1 above)
WPV: One case of wild poliovirus (WPV) is an outbreak; therefore, a targeted healthy
children stool sampling is not recommended.
cVDPV: Circulating vaccine-derived poliovirus (cVDPV) indicate community
transmission; targeted healthy children stool sampling is not recommended.
VDPV genetically linked to another VDPV: The vaccine-derived poliovirus (VDPV) will be
reclassified as a cVDPV; targeted healthy children stool sampling is not recommended.
✓ VDPV not genetically linked to another VDPV: A targeted healthy children stool sampling
may be recommended as part of the initial investigation to determine if there is
community-wide transmission.
○ If a healthy child has a positive VDPV laboratory result and genetic information
indicates it is linked to the VDPV case, this is confirmation of community-wide
transmission. The positive test result is used to reclassify the VDPV case as a
cVDPV case.
○ A positive VDPV result in a healthy child is also used to reclassify an existing
ambiguous vaccine-derived poliovirus (aVDPV) case as a cVDPV case, if viruses
are genetically linked. This is also confirmation of community-wide transmission.
○ If no VDPV is detected among the healthy children, the investigation should
continue to assess if the VDPV case is possibly an immunodeficiency-associated
vaccine-derived poliovirus (iVDPV) or aVDPV case.
Sabin-like 2 (SL2) virus detected within four (4) months of an mOPV2 campaign: SL2
virus detection is expected during an mOPV2 campaign; targeted healthy children stool
sampling is not recommended.
✓ Sabin-like 2 (SL2) virus detected more than four (4) months after last mOPV2 campaign,
or no recent mOPV2 campaign: In these instances, an investigation to the source of the
SL2 virus is warranted – and targeted healthy children stool sampling may be
considered to help guide investigation efforts.
Sabin-like 1 or 3 virus: Detection of Sabin-like 1 and 3 virus is expected given bOPV use
in essential immunization schedules and outbreak response. Targeted healthy children
stool sampling is not recommended.
IMPORTANT: Positive test results from targeted healthy children stool sampling cannot be used as
laboratory evidence of poliovirus in an AFP case (see AFP contact sampling).
When to conduct
Conduct targeted healthy children stool sampling after confirmation that a VDPV is not
genetically linked to another VDPV (i.e., after laboratory test results and sequencing information
are available).
Specimen
labelling
Each specimen should be labelled clearly as a targeted healthy children stool sampling
specimen. The unique identification number should be the same as the positive poliovirus case
with an added targeted healthy children stool sampling indicator (“CC”) and number (#) suffix
(e.g., CC1, CC2, CC3).
“Other”
classification
Positive test results among healthy children are not classified as confirmed poliovirus cases
because they do not meet the case definition, which requires acute flaccid paralysis. Results are
included as “others” in poliovirus isolation counts.
Procedures
Refer to the GPEI Global Polio Surveillance Action Plan 2022–2024 for further details.
aVDPV = ambiguous vaccine-derived poliovirus; bOPV = bivalent oral polio vaccine; cVDPV = circulating vaccine-derived poliovirus;
iVDPV = immunodeficiency-associated vaccine-derived poliovirus; SL2 = Sabin-like type 2; mOPV2 = monovalent oral polio vaccine type
2; VDPV = vaccine-derived poliovirus; WPV = wild poliovirus
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
92
Annex 14. Rapid case and virus detection
Because delays in detection can happen at any stage of field, logistic and laboratory activities, countries
must monitor timeliness at every stage of the process, particularly at the subnational level and
especially in the collection and transport of stool specimens. Only with clear insight into delays can swift
actions be taken to address the identified bottlenecks (Table 14.1). Furthermore, anticipating issues
and proactively identifying alternatives as part of preparedness is highly recommended.
Table 14.1. Delays in detection and possible mitigation measures
Stage
Target
Possible cause
Mitigation measures & solutions
Onset to
care seeking
AFP cases
reported ≤ 7
days of
onset
(ideally
immediately)
● Distance to nearest
facility/person
● Distrust in the health
system
● Cost of service
● Language barrier
● Gender barriers (including
no female nurse/doctor, no
authorization to travel to
health facility)
● Modify data collection tools and analyse by
disaggregated data: social or linguistic profile/at-
risk population group, sex and health-seeking
behaviour.
● Conduct periodic (six-month) social mapping as
part of the active surveillance (AS) network
review to identify gaps in coverage.
● Based on findings, address all issues (e.g.,
mobile clinics, female health workers,
consultation and sensitization with the
community).
Care seeking
to
notification
AFP cases
reported
≤ 7 days of
onset
(ideally
immediately)
● Lack of awareness and
sensitization of healthcare
providers
● Conduct consistent, supportive supervisory visits
to reporting units.
● Ensure training and sensitization of every new
staff member.
● Provide information, education and
communication (IEC) materials: case definition,
reporting requirement and pathway, surveillance
officer contact information.
Notification
to
investigation
< 48 hours
● Lack of training
● Absence of qualified
person to conduct
investigation
● Delay in locating the case
● Case is lost to follow-up
(i.e., cannot find case)
● Competing priorities,
challenging workloads
● Ensure case investigation kits (equipment,
supplies, and materials) are readily available.
● Promote clear responsibilities and reasonable
workloads (i.e., back-up should be available in
the absence of the main surveillance officer).
● Conduct regular trainings for surveillance officers
and back-ups (e.g., other public health staff) at
the field level.
Investigation
to stool 1
collection
< 1 day
● Absence of kit
● Inability to locate the case
(due to discharge, travel,
etc.)
● Case has died
● Ensure case investigation kits (equipment,
supplies and material) are readily available.
● Ensure contact information and address of case
is available.
● If stool specimen collection must be done by
caregiver, ensure it is adequately done.
Stool 1
collection to
stool 2
collection
≥ 24 hours
apart
● Case has died
● Case is no longer at same
location (follow-up issues)
● Provide clear instructions to nurses and
caregivers on collecting the stool specimen.
● Provide clear instructions on contact sampling in
the event of a case of inadequate specimens.
AFP = acute flaccid paralysis; AS = active surveillance; IEC = information, education and communication

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
93
Table 14.1 (continued)
Stage
Target
Possible cause
Mitigation measures & solutions
Stool 2 collection
to shipment to
national level
Stools 1+2 arrival at
laboratory ≤ 3 days of
collection of stool 2
(ideally immediately)
● No or poor
communication on when
stool 2 was collected
● Poor coordination with
courier services
● Issues related to routes
of transport (e.g.,
lockdowns, route
closure)
● Batching of specimens
● Pilot electronic tracking of stool
specimens.
● Plan transport ahead of time,
including plan for contingencies.
● Obtain special permission to
transport samples, if needed.
● Identify alternative routes, carriers.
● Increase storage capacity, identify
storing points.
● Don’t batch specimens.
● Prioritize samples for shipment in
event of suspected polio case
(“hot” case).
Shipment to
national level to
arrival at national
level
Stools 1+2 arrival at
laboratory ≤ 3 days of
collection of stool 2
● Poor planning for
transport, shipment
● Insecurity or road
closures
● Samples kept at national
level until several are
collected and shipped
(“batch” send-off)
● International border
closures
● Suspension of flights
● Pilot electronic tracking of stool
specimens.
● Create contingency plans with
alternative routes or laboratory.
● Explore and pursue ad hoc
solutions in case of conflict or
insecurity (e.g., using humanitarian
flights for transport; sending
samples to an alternative WHO-
accredited lab).
Arrival at national
level to shipment
to (inter)national
laboratory
Stools 1+2 arrival at
laboratory ≤ 3 days of
collection of stool 2
(ideally immediately)
Shipment to
(inter)national
laboratory to
arrival at
(inter)national
laboratory
Stools 1+2 arrival at
laboratory ≤ 3 days of
collection of stool 2
Arrival at
(inter)national
laboratory to final
results
(i.e., negative
results or
sequencing
results for
positive
specimens)
Stools 1+2 are
processed following
standard GPLN
procedures within
defined GPLN target
times for all
procedures
● International border
closures
● Issues with shipping
isolates to sequencing
laboratory
● Shortage of critical
reagents
● Ambiguities in testing
outcomes (e.g.,
mismatched or missing
EPID numbers,
suspicion of cross-
contamination).
● Receipt of large batches
of specimens.
● Ensure a minimum buffer stock
(critical consumables and
reagents) for a one-year workload
when placing orders for 2022.
● Secure a shipping contract with
several in-country couriers.
● Develop an alternative domestic
and international shipping plan with
different sequencing laboratories.
AFP = acute flaccid paralysis; EPID = epidemiological identification; GPLN = Global Polio Laboratory Network; WHO = World Health
Organization

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
94
Annex 15. Polio committees and commissions
While the following terms of reference and descriptions of core activities are generic, groups may take
on additional tasks, depending on current programme needs.
1. The National Polio Expert Review Committee (NPEC or ERC)
The National Polio Expert (Review) Committee (NPEC or ERC), or National Expert Group or National
Polio Expert Panel is an honorary, volunteer group that meets regularly (between once per month to
four times a year). Membership of the committee varies in size. Composition is usually composed of:
• a Chair and a Secretary (usually, the Expanded Programme on Immunization [EPI] manager);
• a paediatrician;
• a neurologist;
• a virologist or microbiologist; and
• an epidemiologist.
The role of the committee is to:
• classify cases of acute flaccid paralysis (AFP) with inadequate specimens that have residual
paralysis at 60-day follow-up or those who either died or were lost to follow-up;
• provide technical advice pertaining to AFP cases and ensure AFP cases have a final diagnosis;
• review cases with adequate specimens and Sabin-like excretion to decide on vaccine-
associated paralytic poliomyelitis (VAPP) diagnosis; and
• monitor quality of the AFP surveillance system in general.
To enable the committee to classify as accurately as possible:
• each case must have accurate, complete investigation in their case investigation form (CIF);
• a copy of the hospital clinical notes or investigations must be included in the case file;
• a copy of the death certificate should be placed in the case file, if the AFP case died;
• a 60-day follow-up form must be included with the district paediatrician’s clinical note; and
• if an AFP case needs to be discussed, the district surveillance team must gather all relevant
documents, bring these to the committee meeting, and present the case.
How to prepare for the committee meeting:
• If the child has monoplegia, arrange for electromyography (EMG) or nerve conduction study
(NCS) to be done before the NPEC meets and bring written results to meeting for discussion.
• Full information should be made available of any underlying conditions or past medical history
that may have bearing on illness causing paralysis.
• A written clinical note from paediatrician describing 60-day follow-up exam with emphasis on
the neurological examination is necessary for most cases.
How to present cases to the committee:
1. History of the illness
o Presence of fever and other symptoms at onset
o Description of progression of illness
o Hospital course, including investigations results
2. Exam of child at initial presentation
o Description of general physical exam
o Site and extent of weakness
o Reflexes and tone
3. Exam of child at 60-day follow-up exam
o Detailed neurological exam
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
95
2. National Certification Committee (NCC)
National Certification Committees (NCCs) are groups of independent experts in disciplines relevant for
the certification of polio eradication, such as public health, immunization, epidemiology, paediatrics,
infectious diseases, neurology and virology. NCCs are appointed by the national government in
consultation with regional offices of the World Health Organization (WHO). NCC members act in a
personal capacity only and cannot have responsibility for any activities to implement polio eradication in
the country.
NCCs are responsible for assessing and verifying national documentation on polio-free status, which is
assembled by the Ministry of Health (MoH) with WHO support. NCCs cannot certify polio eradication in
their country, which is the role of the Regional Certification Commission (RCC) and Global Certification
Commission for the Certification of the Eradication of Poliomyelitis (GCC) in review of NCC-supporting
documentation on the polio-free status of the country.
Certification, which is done at the regional level, requires the absence of WPV transmission from any
source (AFP, community samples and sewage samples) for at least three (3) consecutive years and a
timely and sensitive AFP surveillance that meets the GCC’s certification standards and the following
performance indicators:
30
- Detection of at least one (1) NPAFP case annually per 100 000 children younger than 15 years.
- Collection of adequate stool specimens from at least 80% of AFP cases.
- Testing of all specimens at a WHO-accredited laboratory.
In WHO regions not yet certified as wild poliovirus (WPV)-free and for Member States where no WPV
has been detected from any source for at least three (3) years under conditions of “certification-
standard” surveillance, NCCs provide the RCC with documentation on all aspects related to polio
eradication, including immunization activities, surveillance (including environmental surveillance of
polio-essential facility [PEF] wastewater), laboratory support, and containment.
Once the RCC formally accepts this documentation, signaling their agreement with the NCCs claim that
WPV transmission in the country has been interrupted, the NCC will continue to provide annual reports
to the RCC on the maintenance of polio-free status in the country.
Each NCC in their role also conveys recommendations on how to improve polio activities from the RCC
to their national government.
3. Regional Certification Commissions (RCCs)
RCCs are independent panels of international public health experts advising the WHO on all issues
related to the certification of WPV eradication at the regional level. RCCs have the authority to certify
the eradication of indigenous WPV in the region after considering all necessary evidence, including the
views of NCCs and results of field visits to countries.
In WHO regions not yet certified as WPV-free, RCCs monitor progress towards interrupting WPV
transmission and will eventually certify the WHO region as free of wild WPV, provided that a period of at
least three (3) years have passed without identification of WPV.
31
In WHO regions already certified as WPV-free, RCCs annually review updated documentation from
each Member State on the maintenance of WPV-free status, i.e., on immunization, surveillance, polio
laboratory support and poliovirus containment. RCCs then report conclusions on risk assessment and
any risk mitigation measures to the respective country and WHO Regional Director. Related to
poliovirus containment, RCCs in certified regions work with NCCs to review national reports and
documentation, specifically updating and maintaining complete inventories of facilities which previously
hosted WPV or any other infectious or potentially infectious poliovirus materials.
30
Given programme advancements in genomic analysis and the widespread use of environmental surveillance in many countries,
the GCC is reviewing the criteria and may recommend certification sooner than the traditional three years. Changes to these
requirements will be posted on the GPEI website (https://polioeradication.org/polio-today/preparing-for-a-polio-free-
world/certification).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
96
4. Global Commission for the Certification of the Eradication of Poliomyelitis (GCC)
The GCC is the independent global oversight body which will issue, if and when appropriate, a final
report to the Director-General of the WHO (DG-WHO) to certify that the global eradication of WPV has
been achieved. The GCC also oversees global poliovirus containment. It receives annual reports from
RCCs on poliovirus survey and inventory activities in all six WHO regions, as reported by NCCs in their
annual reports to the RCCs on the achievement or maintenance of WPV-free status.
The GCC is expected to eventually certify that global containment of all retained live poliovirus
materials—including WPV, Sabin and vaccine-derived poliovirus (VDPV) of all types—has been
achieved and maintained. It is still yet to be decided whether the GCC will exist by the time containment
of all poliovirus materials (WPV, Sabin and VDPV) will be achieved. As of this writing, the mandate to
the GCC from the DG-WHO remains to certify WPV eradication.
As of 2022, five of six WHO regions have been certified wild poliovirus free; however, as long as wild
poliovirus is not eradicated globally, NCCs and RCCs still have also a role to play in monitoring polio
surveillance performance in their respective country and in updating the GCC.
For additional information on certification, refer to GPEI webpage on Preparing for a Polio-Free World
(https://polioeradication.org/polio-today/preparing-for-a-polio-free-world/certification).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
97
Annex 16. Safety surveillance for nOPV2
Countries facing outbreaks of circulating vaccine-derived poliovirus type 2 (cVDPV2) may opt to use the
novel oral polio vaccine type 2 (nOPV2), available under an Emergency Use Listing (EUL) of the World
Health Organization (WHO). A required component for the deployment and post-deployment of nOPV2
is safety monitoring to detect possible adverse events that may occur following immunization.
Two kinds of adverse events are monitored through vaccine safety surveillance:
1. Adverse event following immunization (AEFI) is defined as any untoward medical
occurrence which follows immunization and does not necessarily have a causal relationship
with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign,
abnormal laboratory finding, symptom or disease.
2. Adverse event of special interest (AESI) is defined as a pre-identified and predefined
medically significant event that has the potential to be causally associated with a vaccine
product and needs to be monitored. AESIs of interest for nOPV2 surveillance are:
o anaphylactic reactions;
o aseptic meningitis / encephalitis;
o acute disseminated encephalomyelitis (ADEM);
o Guillain-Barré syndrome (GBS) / Miller Fisher syndrome;
o myelitis / transverse myelitis;
o acute flaccid paralysis (AFP) due to vaccine-derived poliovirus (VDPV) or vaccine-
associated paralytic poliomyelitis (VAPP); and
o unexplained deaths.
Surveillance activities for nOPV2 adverse events
Passive and active AEFI surveillance: Passive AEFI surveillance (spontaneous reporting) detects
AEFIs for all vaccines, including nOPV2, and typically follows a process of case identification,
notification, reporting, investigation and causality assessment separate from the AFP surveillance
system. Active AEFI surveillance detects more complex adverse events that may be anticipated and, for
nOPV2, include AFP surveillance and active AESI surveillance, both of which require the active
involvement of the country’s immunization and surveillance programmes (Fig. 16.1).
Active AESI surveillance: AESI surveillance is conducted in sentinel sites (see nOPV2 AESI guidance
among sources listed below). Matching the clinical and laboratory findings with prespecified case
definitions is important for AESI case confirmation. AESI surveillance continues for six (6) weeks
following each nOPV2 campaign and focuses on children in the eligible age range for nOPV2.
National Regulatory Authorities (NRAs) and national immunization programmes are typically involved in
the reporting structure for AEFI/AESI, and a national Vaccine Safety Advisory Committee (or Causality
Assessment Committee) reviews the data on serious adverse events to conduct the causality
assessment which determines the likelihood that an event was caused by a vaccine or vaccination (Fig.
16.1). The data generated from these surveillance systems are shared with the WHO for monitoring and
assessment by the Global Advisory Committee on Vaccine Safety (GACVS) to verify continued safety
of the vaccine and to support its full listing through the WHO prequalification process.
Requirements for nOPV2 safety surveillance
Prior to nOPV2 rollout, countries need to meet the following safety monitoring requirements:
1. Confirmation of nOPV2 safety surveillance monitoring activities including:
a) a national AEFI surveillance manual or abridged guide and key forms; and
b) an active AESI safety monitoring protocol for nOPV2 (if applicable – see below)
2. An operational plan for implementing nOPV2 safety surveillance, which includes:
a) plans for implementing AEFI surveillance;
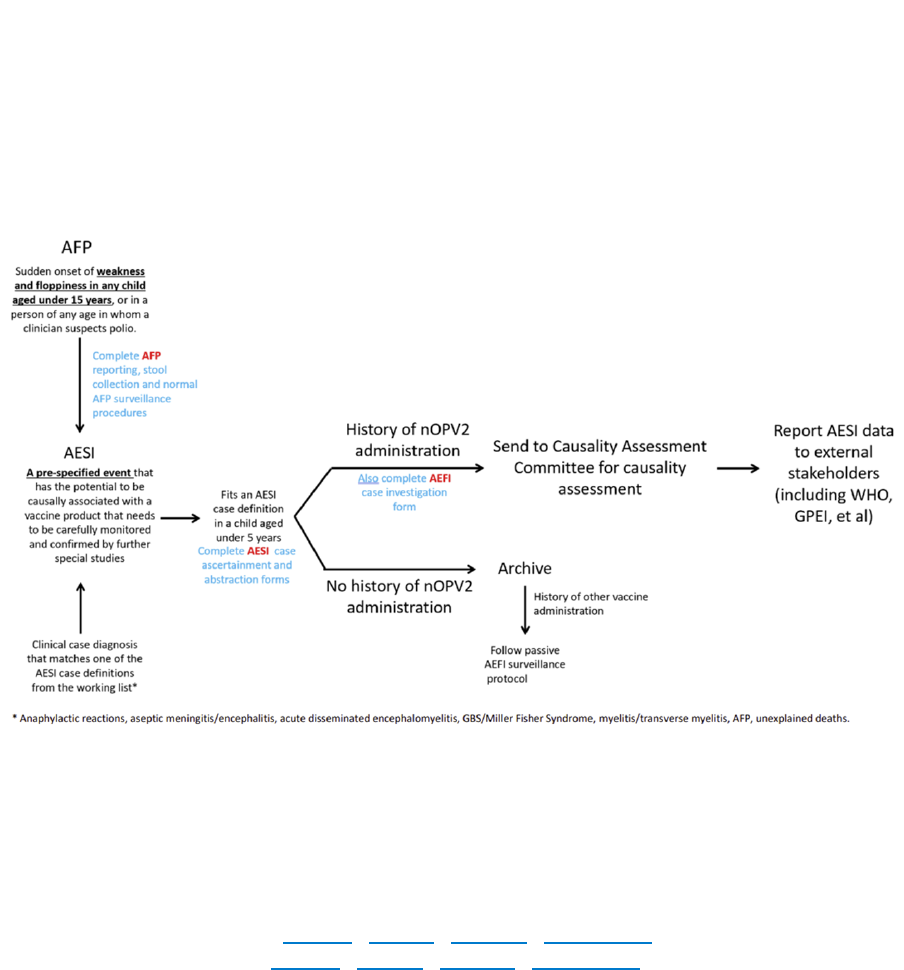
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
98
b) plans for managing a vaccine-related event (VRE); and
c) confirmation of data sharing processes and timelines.
3. Key nOPV2-related safety trainings have been completed or are planned.
4. Causality Assessment Committee terms of reference (TORs) and list of members, training
plans and, if applicable, previous committee meeting minutes.
Recommendations for nOPV2 safety surveillance as it relates to AFP surveillance
GACVS recommends that active AESI surveillance for nOPV2 safety be implemented but does not
require it for countries without sufficient technical capacity and human resources to implement the
active AESI protocol. In these countries, detection of AESI and potentially other safety signals should
build upon the ongoing AFP, passive AEFI, and environmental surveillance systems, and close
monitoring of VREs. AFP surveillance thus remains the critical source of safety data for the
nOPV2 vaccine.
Fig. 16.1. Active AFP and AESI surveillance after nOPV2
AESI = adverse event of special interest; AFP = acute flaccid paralysis; GPEI = Global Polio Eradication Initaitive; nOPV2 = novel oral
polio vaccine type 2; WHO = World Health Organization
Source: GPEI, Guide for Surveillance of Adverse Events of Special Interest (AESI) during Novel Oral Polio Vaccine Type 2 (nOPV2) Use.
nOPV2 safety surveillance materials
1. nOPV2 Safety Guidance | English | French | Russian | Portuguese |
2. nOPV2 AESI Guidance | English | French | Russian | Portuguese |
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
99
Annex 17. Surveillance activities in outbreak settings
The following is a checklist of surveillance strengthening activities during a poliovirus outbreak. Details
are included in Quick Reference on Strengthening Polio Surveillance during a Poliovirus Outbreak.
AFP surveillance
✓ Immediately notify surveillance and laboratory personnel upon polio outbreak confirmation.
✓ Increase the annualized target non-polio acute flaccid paralysis (NPAFP) rate to > 3 per 100,000
children <15 years old per year.
✓ All districts and provinces should review and update (if necessary) their polio surveillance reporting
network, including prioritization of reporting sites for active surveillance visits.
✓ Ensure active surveillance (AS) visits are conducted regularly and monitored nationwide.
✓ Ensure that routine (passive) surveillance is performing optimally.
✓ Conduct facility-based, ad hoc active case searches to identify any unreported cases of acute
flaccid paralysis (AFP).
✓ Use all opportunities for community-based, ad hoc active case searches to find unreported cases.
✓ Verify that special populations within the outbreak-affected and high-risk areas are included in
surveillance activities and implement tailored approaches, as necessary.
✓ Ensure supportive supervision and monitoring of surveillance officers are conducted.
✓ Monitor surveillance performance and use data for action.
✓ Prioritize investigation of silent districts or provinces within the outbreak-affected or high-risk areas.
✓ Establish regular review meetings among AFP surveillance partners.
AFP case investigation
✓ Collect key information that may not be included in the AFP case investigation form.
✓ Conduct AFP contact sampling for all AFP cases with inadequate stool specimens and consider
expanding AFP contact sampling for all AFP cases in certain outbreak and polio high-risk settings.
✓ Prioritize 60-day follow-up investigations for AFP cases with inadequate stool specimens.
Sensitization activities
✓ Conduct re-fresher trainings on polio and polio surveillance for surveillance officers and teams.
✓ Conduct AFP surveillance sensitization activities among healthcare providers.
✓ Conduct polio and AFP surveillance sensitization activities among communities.
✓ Conduct polio and AFP surveillance sensitization activities among governmental and
nongovernmental organizations and engage their support.
Environmental surveillance
✓ Determine the adequacy of existing environmental surveillance (ES) sites.
✓ Identify high-risk areas for ES expansion during an outbreak, including ad hoc ES sites.
Laboratory surveillance
✓ Establish regular, ongoing communication among surveillance and laboratory staff at all levels.
✓ Prioritize stool specimen and sewage sample testing from outbreak-affected and high-risk areas.
✓ Verify that stool specimens and sewage samples are collected as recommended and reverse cold
chain is maintained from point of collection to arrival at a WHO-accredited laboratory.
✓ Adjust stool and sewage sample transport networks, as necessary, to ensure a well-coordinated
and rapid delivery system is maintained.
✓ Ensure laboratory resources are available to meet the demand of increased testing – and have a
contingency plan available in case it cannot.
Additional considerations
✓ Targeted healthy children stool sampling has limited use for strengthening polio surveillance.
✓ Include surveillance updates in the national Polio Outbreak Situation Report (SitRep).
✓ Prepare for GPEI’s Outbreak Response Assessment (OBRAs).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
100
Annex 18. Gender and polio surveillance
The Global Polio Eradication Initiative (GPEI) published its Gender Equality Strategy 2019–2023 to
provide both direction and scope for advancing equality and for strengthening gender mainstreaming
across all interventions, strategies and policies.
31
Surveillance programme and staff should be alerted to:
• gender-related barriers in surveillance detection and response; and
• gender equality in the work environment and organizational culture
Gender-related barriers in surveillance detection and response
In any context and especially in high-risk areas and with special populations, the polio surveillance
system must be able to identify the stages at which gender norms, roles and relations, as well as
existing gender inequalities, may affect case detection and notification (Table 18.1).
To minimize the risk of gender-related delays in detection:
• programmes are encouraged to collect and analyse sex-disaggregated data on a systematic
basis, including through adapted case investigation forms (CIFs) and analytic tools, and identify
stages with consistent, recurrent (over a 12- to 24-month period) delays in detection,
notification and investigation that may be linked to gender barriers; and
• where observed, surveillance officers and/or programme managers should conduct in-depth
assessments with the support of the management and gender specialists and consider
possible, locally acceptable actions to address the gaps (Table 18.1).
When considering actions to inform and support surveillance interventions, always:
• collaborate with and reach out to women's groups, women's health committees, grassroots
networks and other organizations with a strong understanding and influence around health-
seeking behaviours, gender-related barriers and children's health issues;
• consult with community authorities, religious leaders, opinion influencers, and elders, including
women, to sensitize and negotiate access to women or households and increase women’s
participation;
• sensitize and promote fathers’ and men’s equal participation in childcare, caregiving, and
household responsibilities and tasks; and
• ensure communication channels, tools, materials, and messages are context-specific, informed
by gender analysis, and free from harmful gender stereotypes.
31
Global Polio Eradication Initiative (GPEI). Gender Equality Strategy 2019–2023. Geneva: World Health Organization; 2019
(https://polioeradication.org/wp-content/uploads/2020/10/Gender-Strategy.pdf). See also GPEI Gender and Polio Eradication
[website]. Geneva: World Health Organization; 2021 (https://polioeradication.org/gender-and-polio/gender-and-polio-eradication).
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
101
Table 18.1. Examples of gender-related barriers in surveillance detection and response
Stage
Possible issues & their causes
Proposed possible actions
Onset of
paralysis to
care-seeking
Not seeking care or delay in seeking care due to:
● women caregivers lack decision-making power
and/or faces challenges or restrictions in mobility
(lack of transport, money, time, multiple
household duties, need of authorization to travel
to health facility, and/or of a male
escort/traveling companion)
● low awareness and literacy rate of women
caregivers and lack of access to health
information in suitable formats
● discriminatory attitude in health-seeking
behaviour for female patients (e.g., boys’ access
to health care prioritized / delays in seeking care
for girls, poor quality of services of health
workers towards women)
● absence of local female healthcare providers
Carry out gender analysis/assessment to
identify specific gender barriers to the
context/setting.
● Advocate with local authorities.
● Sensitize community and involve men in
sensitization and outreach activities.
● Adapt services to women’s need (adapt
opening times for health services, outreach
surveillance activities, etc.).
Notification
Late or no notification due to:
● insufficient knowledge and training opportunities
provided for women care worker
● unresponsive medical hierarchy when a female
worker notifies an AFP case
● active surveillance visits not conducted regularly
and/or adequately due to lack of suitable modes
of transport, and/or male escort
● lack of women as community informants (e.g., in
CBS) due to existing gender norms and roles
● Ensure availability of training for all staff.
● Engage with women workers at the
forefront to identify and address their needs
and challenges, esp. safety related (e.g.,
timing of trainings, transport options,
location).
● Sensitize local health workers (including to
security/safety considerations).
● Ensure availability of safe and adequate
transport for personnel.
● Reaching out to and collaborating with local
women’s groups to find solutions.
● Adjust CBS team composition.
Case
investigation
and stool
collection
Delayed investigation and/or stool collection due to:
● insufficient training opportunities provided for
women surveillance officers
● lack of female surveillance officers needed to
enter home of AFP case
● inability of women caregivers to stay overnight in
a health facility when case is hospitalized
● safety and security risks faced by women
workers
● Training of healthcare worker/surveillance
officers takes into account gender-related
challenges and barriers to women’s
participation (e.g., location, timing,
transport, traveling companion if needed).
● Adjust surveillance team composition.
● Sensitize local health system and/or
community.
● Ensure safety of women working at the
forefront.
AFP = acute flaccid paralysis; CBS = community-based surveillance
Gender equality in the work environment, and organizational culture
Managers of polio surveillance must ensure that a gender lens is also applied to the programme both to
promote gender equality and to address any gender-related barriers or other factors impacting the
safety and performance of its staff, as well as their career advancement. Below are actions to consider.
Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
102
• Institutionalize the systematic and regular provision of gender analysis in all reports.
• Increase women's equal and meaningful participation in surveillance, including a gender balance
among supervisors, and identify gaps in team composition that contribute to deficiencies in case
investigation (e.g., all-male teams not being able to access homes in certain contexts).
• Identify specific needs and barriers faced by women frontline workers (e.g., needs or barriers
related to safety, mobility/transportation, literacy [including digital literacy], and training).
• Ensure that the gender module is included in all polio surveillance trainings, with a focus on a
description of gender and gender-related barriers in surveillance. Also conduct mandatory staff
training on preventing and responding to sexual exploitation, abuse and harassment (PRSEAH).
• Share information about existing reporting and support mechanisms and systems in place to
address all forms of sexual exploitation, abuse or harassment. If not already in place, set up
communication mechanisms for women involved in polio surveillance to be able to voice and
discuss in confidence those issues impacting their physical and emotional wellbeing at work (e.g.,
mentorship, staff representative).
• Ensure that training and sensitization sessions at health facilities or within communities:
• include gender-related barriers to immunization and surveillance;
• highlight equal parenting, shared caregiving responsibilities and fathers’ equal participation in
childcare, caregiving and household tasks (preferring the words “parents and caregivers”);
• try to ensure that diverse women and men are represented in training visuals and images;
• provide sex-disaggregated data and gender analysis whenever possible, with “real life”
examples and illustrations, and highlight the importance of collecting and analysing data
disaggregated by sex in all monitoring and evaluation (M&E) activities (Table 18.2); and
• are accessible to all participants (e.g., facilities are safe and easily reached, timing is
accommodating, seating arrangement is appropriate, and organizers and facilitators know how
to facilitate sessions to ensure participation from all).
Table 18.2. Gender-related indicators
Indicators
Calculation (expressed as a percentage)
Case detection
# of AFP cases** by sex with final lab results ≤35 days of onset
/
# of AFP cases
Timeliness of
field activities
# of AFP cases by sex with 2 samples collected ≥ 24 hrs apart, both within 11 days of
paralysis onset
/
# of reported AFP cases
Timeliness of
notification
# of AFP cases by sex reported within 7 days of paralysis onset
/
# of reported AFP cases
Health contact
# of AFP cases by sex with ≤2 healthcare encounters between onset and before notification
/
# of AFP cases
Professional
profile by sex
(by category)
# of women [professional profile]
/
total # of staff or informants (by category: surveillance officer, supervisor, CBS informant)
Staff with
completed
PRSEAH
# of surveillance staff having completed PRSEAH training
/
# of staff
AFP = acute flaccid paralysis; CBS = community-based surveillance; PRSEAH = preventing and responding to sexual exploitation, abuse,
and harassment
**Aggregated results: all lab results (AFP + contacts) used to classify AFP case as confirmed/discarded

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
103
Annex 19. Scientific resources
Table 19.1. Resources to support surveillance for acute flaccid paralysis (AFP)
Focus area
Resources
Programme
information
● Global Polio Eradication Initiative (GPEI): polioeradication.org
● The GPEI website includes updated global counts on wild and vaccine derived poliovirus
cases.
For additional polio publications on topics such as surveillance, outbreaks, and testing, as
well as special topics such as on containment, visit the following website:
● Morbidity and Mortality Weekly Report (MMWR): www.cdc.gov/mmwr/index.html
● Weekly Epidemiological Record (WER): www.who.int/wer/en
AFP
surveillance
● Global Polio Surveillance Action Plan 2022-2024
polioeradication.org/wp-content/uploads/2022/04/GPSAP-2022-2024-EN.pdf
● Global Polio Surveillance Action Plan 2018-2020
polioeradication.org/wp-content/uploads/2016/07/GPEI-global-polio-surveillance-action-
plan-2018-2020-EN-1.pdf
● Quick Reference on Strengthening Polio Surveillance during a Poliovirus Outbreak
polioeradication.org/wp-content/uploads/2021/12/Quick-Reference_Strengthening-
Surveillance-during-Poliovirus-Outbreaks_24-March-2021.pdf
● Guidelines for Implementing Polio Surveillance in Hard-to-Reach Areas & Populations
polioeradication.org/wp-content/uploads/2020/10/Guidelines-polio-surveillance-H2R-
areas.pdf
● Job Aid: Use of AFP contact sampling and targeted healthy children stool sampling
polioeradication.org/wp-content/uploads/2020/03/AFP-contact-sampling-and-targeted-
healthy-children-stool-sampling-20200327.pdf
● Best practices in active surveillance for polio surveillance
polioeradication.org/wp-content/uploads/2018/12/Best-practices-in-active-surveillance-
for-polio-eradication.pdf
● Guidelines for Implementing Poliovirus Surveillance among Patients with Primary
Immunodeficiency Disorders (PIDs)
polioeradication.org/wp-content/uploads/2020/12/Guidelines-for-Implementing-PID-
Suveillance-3.3-20201215.pdf
● Classification and reporting of vaccine-derived polioviruses (VDPV).
polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-
VDPVs_Aug2016_EN.pdf
● Standard Operating Procedures: Responding to a Polio Event or Outbreak
polioeradication.org/wp-content/uploads/2022/07/Standard-Operating-Procedures-For-
Responding-to-a-Poliovirus-Event-Or-Outbreak-20220807-EN-Final.pdf
● Polio Field and Laboratory Surveillance Requirements in the Context of nOPV2 use
polioeradication.org/wp-content/uploads/2022/06/nOPV2-surveillance-guidance.pdf
● Interim guidance for the poliomyelitis (polio) surveillance network in the context of
coronavirus disease (COVID-19)
www.who.int/publications/i/item/WHO-POLIO-20.04
Community-
based
surveillance
● Technical Contributors to the June 2018 WHO meeting. A definition for community-based
surveillance and a way forward: results of the WHO global technical meeting, France, 26
to 28 June 2018. Euro Surveill. 2019;24(2): pii=1800681.
doi.org/10.2807/1560-7917.ES.2019.24.2.1800681
Poliovirus testing
● Department of Immunization, Vaccines, and Biologicals (2004) WHO Polio Laboratory
Manual 4th ed. Geneva, Switzerland: World Health Organization. WHO/IVB/04.10.
apps.who.int/iris/bitstream/handle/10665/68762/WHO_IVB_04.10.pdf

Global guidelines for acute flaccid paralysis (AFP) surveillance in the context of poliovirus eradication
104
Gender training
● Gender and Polio Introductory Training: Facilitation Guide
polioeradication.org/wp-content/uploads/2022/06/Gender-and-polio-introductory-training-
facilitation-guide-20220620.pdf
● Gender and Polio Introductory Training: Presentation Slides
polioeradication.org/wp-content/uploads/2022/06/Presentation-Gender-and-Polio-
Training.pdf
● Gender and Polio profile
polioeradication.org/wp-content/uploads/2022/06/Gender-and-Polio-Profile-20220620.pdf
VPD surveillance
● Surveillance standards for vaccine-preventable diseases, 2nd ed. Geneva: World Health
Organization; 2018.
www.who.int/publications/i/item/surveillance-standards-for-vaccine-preventable-diseases-
2nd-edition
● Global strategy for comprehensive Vaccine-Preventable Disease (VPD) surveillance.
www.who.int/publications/m/item/global-strategy-for-comprehensive-vaccine-preventable-
disease-(vpd)-surveillance
