
Plan Sponsor Notice:
Planning for the COVID-19 Emergencies Ending on May 11
On January 30, 2023, the federal government announced that the two national emergencies addressing
COVID-19, the public health emergency (PHE) and the national emergency, will end on May 11, 2023.
Starting May 12, 2023, health plans and group plan sponsors will no longer be subject to federal
requirements for coverage of COVID-19 testing, vaccinations and treatments.
Below is a summary of the changes to COVID-19 coverage that will take place once the federal
emergencies end for self-funded plan sponsors.
Please note that, as a self-funded plan sponsor, you may elect to extend coverage through the end of
2023 or the end of your plan year. If you would like to consider that, please contact your account team
by March 31, 2023.
Aetna® plan sponsors who have carved out pharmacy benefits to a third-party administrator will need to
consult their Pharmacy Benefit Manager on all benefits other than lab-based COVID-19 tests (covered
under the medical plan benefit), and the COBRA, HIPAA and Special Enrollment Period deadline changes.
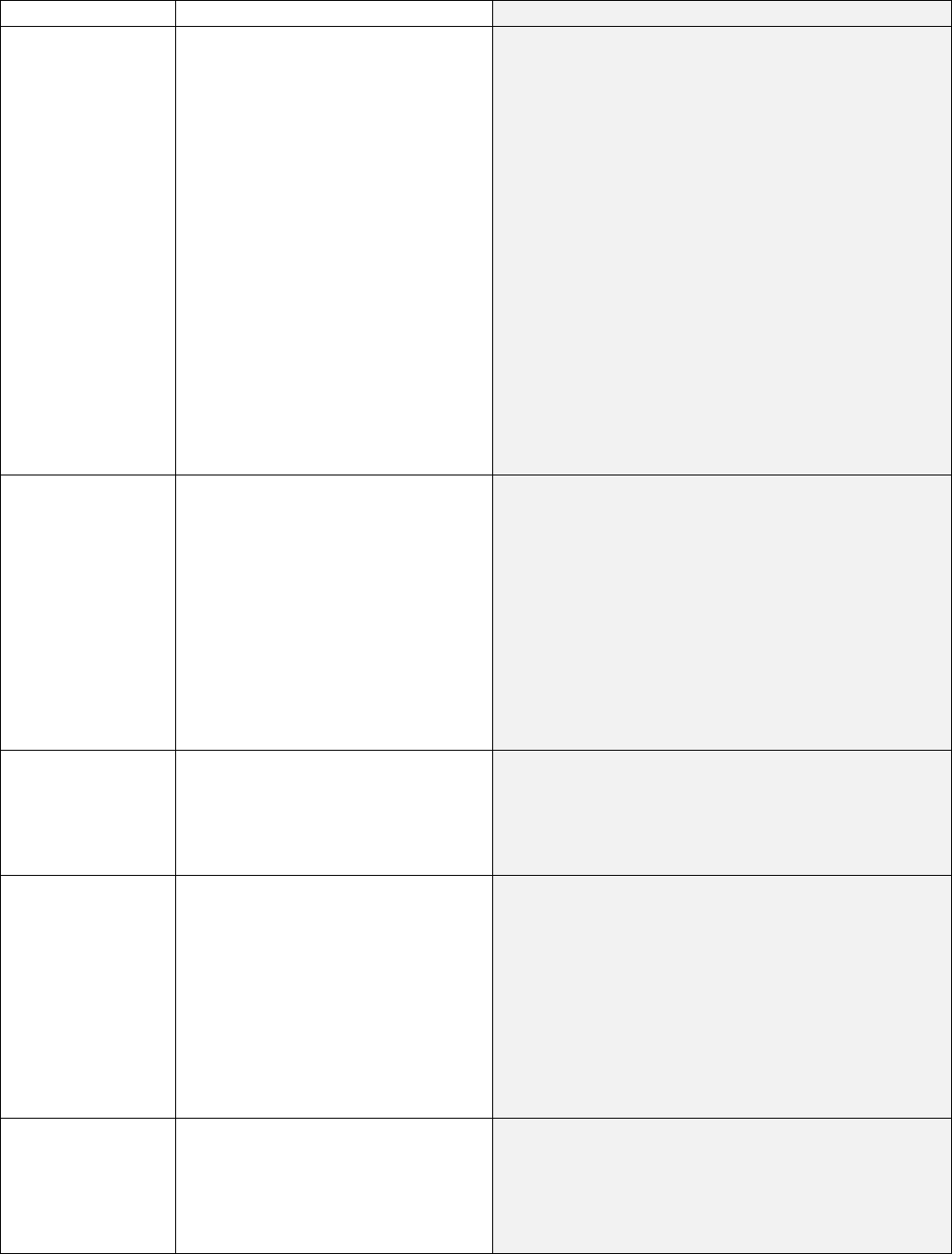
During the emergency period
Starting May 12, 2023
COVID-19
vaccines,
including
boosters
Members pay $0 for the vaccine at
any location.
The government covers the
ingredient cost of the COVID-19
vaccine and requires plans to cover
all vaccines at 100%, both in and
out of network.
Members will pay $0 for the vaccine at in-network
locations.*
Many pharmacies now have ample supply of the
government funded vaccines.
Although timing is unknown, it is likely that COVID-
19 vaccines will be commercially available in the
summer or fall. At that time, we expect the COVID-
19 vaccine will be added to the Aetna standard
seasonal vaccine program.
Note that, once federal supply is exhausted, the
vaccines will continue at 100% coverage for in-
network administration. Normal plan cost share will
apply for out-of-network administration, like the flu
vaccine.
COVID-19 at-
home test kits,
also known as
over-the-counter,
or OTC test kits
Members pay $0 for select test kits.
Plans cover eight OTC COVID-19
tests per month with a $0 member
cost share, if obtained at a
pharmacy, or with a post-service
reimbursement claim.
Members will pay the retail cost of test kits. They
are no longer covered.
Members will be able to get an at-home test kit for
around $12 per test, or $24 for a box of two from
CVS® and other retailers.
Members can also use funds from a health savings
account or a flexible spending account toward test
kits.
COVID-19 lab
tests
Members pay $0 for lab tests,
including rapid diagnostic and
swab-and-send tests, at in-network
locations.
Members will pay their copay, coinsurance or
deductible at in-network locations. It will be applied
to their out-patient testing benefit, which is part of
their medical plan.
Evaluation &
Management
Visit (E&M) -
Telemedicine,
Urgent Care, ER
and Office Visits
Associated with
COVID-19 tests
Members pay $0 for COVID-19
associated visits (INN and OON)
when there’s an associated COVID-
19 test done within 2 days before
or 2 days after.
Members will pay their copay, coinsurance or
deductible for COVID-19 associated visits (INN and
OON) when there’s an associated COVID-19 test
done within 2 days before or 2 days after.
COVID-19 anti-
viral medications
or treatments,
like Paxlovid*
Members pay $0 for these
prescriptions.
No change. Members will pay $0 for these
prescriptions while the government supply is
available.
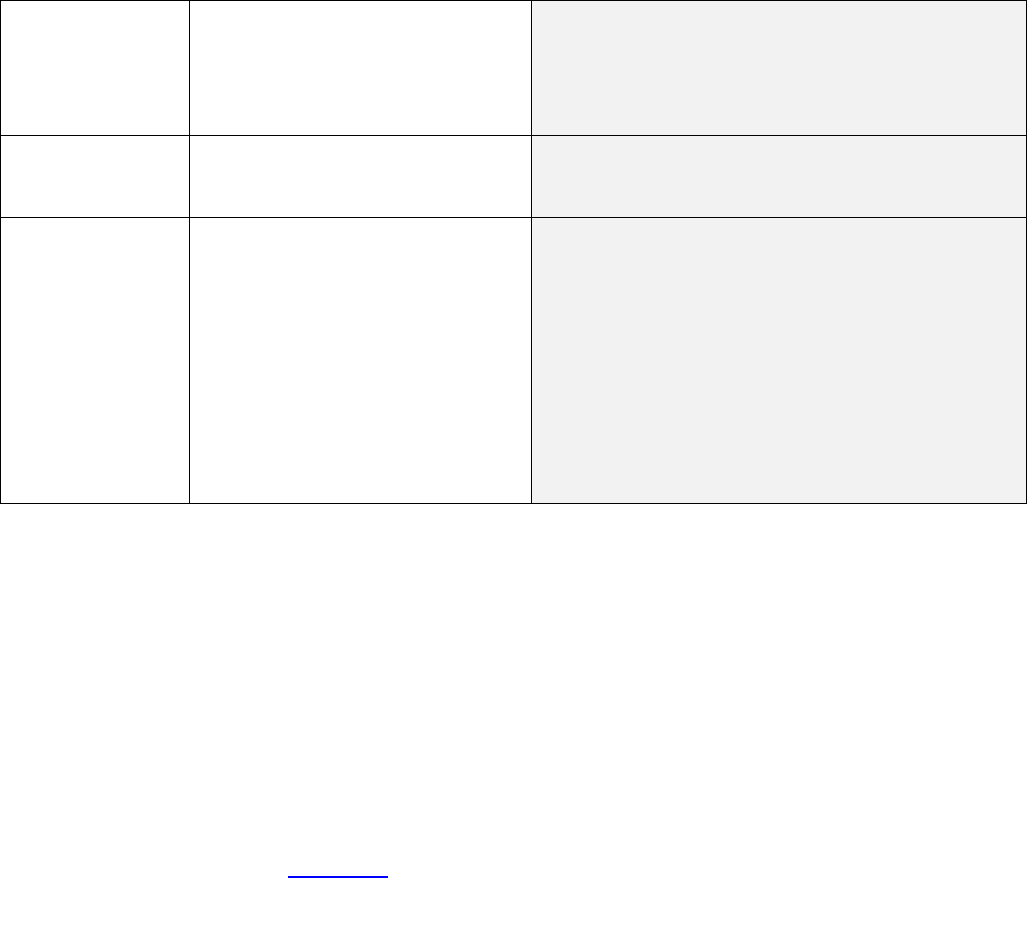
Pharmacist
Assessment and
Prescribing of
Paxlovid program
For plan sponsors with this
program, members pay $0 for
pharmacist assessment and
prescribing of Paxlovid at
pharmacies, including CVS.
No change.
For plan sponsors with this program, coverage will
continue, consistent with their current benefits.
COVID-19
monoclonal
antibodies
Members pay normal cost sharing
for EUA-approved monoclonal
antibody treatments.*
No change. Members will continue to pay normal
cost sharing for EUA-approved monoclonal antibody
treatments.*
COBRA, HIPAA,
special
enrollment and
benefit claims
and appeals
The national emergency extended
deadlines for:
• COBRA elections
• Paying COBRA premiums
• Electing HIPAA special
enrollment
• Filing claims, appeals and
requests for external
review
Deadlines return to normal timeframes starting July
10, 2023.
*Under an EUA declaration, the FDA may authorize unapproved medical products or unapproved uses of
approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-
threatening diseases or conditions.
Certain state COVID-19 requirements may have expiration dates that are not tied to the end of the
federal emergencies. Aetna will follow all federal and state mandates, as required.
We will continue to provide updates if needed.
Sincerely,
Your Aetna Account Team
Policies and plans are insured and/or administered by Aetna Life Insurance Company or its affiliates
(Aetna). Providers are independent contractors and are not agents of Aetna. Provider participation may
change without notice. Refer to Aetna.com for more information about Aetna® plans. Aetna and CVS
Pharmacy® are part of the CVS Health® family of companies. For a complete list of other participating
pharmacies, log in to Aetna.com and use our provider search tool.
©2023 Aetna Inc.
1979000-01-01 (3/23)
