
J.
Phy8iol.
(1965),
176,
pp.
337-354
337
With
8
text-ffgure8
Printed
in
Great
Britain
THE
RATE
OF
TENSION
DEVELOPMENT
IN
ISOMETRIC
TETANIC
CONTRACTIONS
OF
MAMMALIAN
FAST
AND
SLOW
SKELETAL
MUSCLE
By
A.
J.
BULLER
AND
D.
M.
LEWIS
From
the
Physiology
Department,
King's
College,
London
(Received
8
June
1964)
Following
the
demonstration
by
Buller,
Eccles
&
Eccles
(1960b)
that
the
speed
of
contraction
of
mammalian
skeletal
muscles
was
at
least
partially
determined
by
the
motor
nerve
innervation,
two
central
problems
re-
mained.
First,
what
part
or
parts
of
the
contractile
machinery
of
the
muscle
are
influenced
by
the
motor
innervation,
and,
secondly,
how
do
the
motoneurones
bring
about
their
influence
upon
the
muscle
fibres?
The
solution
of
the
first
of
these
two
questions
requires
a
more
detailed
study
of
the
contractile
mechanism
of
mammalian
muscle
than
has
hitherto
been
made,
with
particular
attention
to
any
differences
which
exist
between
fast
and
slow
skeletal
muscle.
In
the
mammal,
both
the
fast
and
slow
skeletal
muscles
consist
of
twitch
fibres,
and
both
types
of
muscle
are
therefore
comparable
with
the
fast
fibre
system
of
the
frog.
Only
very
recently
has
there
been
a
demonstration
of
the
equivalent
of
the
frog
slow
fibre
system
in
mammalian
muscles,
and
as
yet
such
fibres
have
only
been
identified
in
extrinsic
ocular
muscles
(Hess
&
Pilar,
1963).
The
present
paper
is
concerned
with
an
investigation
into
the
rate
of
isometric
tension
development
in
mammalian
fast
and
slow
muscles
following
repetitive
stimulation
of
their
motor
nerves.
This
study
was
a
necessary
preliminary
to
the
understanding
of
the
alterations
which
occur
in
the
rate
of
tension
development
following
operative
cross
union
of
the
nerves
to
mammalian
fast
and
slow
muscle
(Buller
&
Lewis,
1964,
1965b).
A
preliminary
account
of
some
of
the
experiments
herein
reported
has
already
been
published
(Buller
&
Lewis,
1963
a).
METHODS
The
experiments
were
performed
on
cats
weighing
between
1-8
and
2-8
kg
anaesthetized
with
pentobarbitone
sodium
(Nembutal).
An
initial
dose
of
40
mg/kg
was
injected
intra-
peritoneally,
and
anaesthesia
was
maintained
by
subsequent
intravenous
injections
through
a
jugular
cannula.
After
making
an
incision
down
the
mid
line
of
the
calf,
the
muscles
to
be
studied
were
dissected
free
from
surrounding
structures
whilst
still
preserving
their
full
blood
supply.
While
other
hind-limb
muscles
have
been
examined
this
paper
will
confine
itself
to
a

A.
J.
BULLER
AND
D.
M.
LEWIS
comparison
of
the
soleus
and
flexor
hallucis
longus
muscle.
This
latter
muscle
is
more
correctly
called
the
mesial
head
of
the
flexor
digitorum
loingus
(Chin,
Cope
&
Pang,
1962),
since
in
the
cat
its
tendon
of
insertion
fuses
with
that
of
the
larger
flexor
digitorum
longus.
In
this
paper,
however,
we
shall
retain
the
commoner
terminology
of
flexor
hallucis
longus
(F.H.L.).
Soleus
and
F.H.L.
muscles
were
chosen
because
they
are
the
slowest
and
fastest
con-
tracting
calf
muscles
respectively
and
because,
in
any
one
cat,
their
maximum
tetanic
tensions
rarely
differ
by
more
than
30
O0.
The
individual
motor
nerves
to
the
muscles
to
be
used
were
carefully
dissected
to
allow
an
ample
length
for
stimulation.
They
were
then
cut
centrally
and
the
distal
ends
prepared
for
mounting
on
bipolar
stimulating
electrodes.
The
individual
muscle
tendons
were
very
firmly
tied
to
steel
hooks
which
could
be
attached
directly
to
the
strain
gauge.
The
length
of
tendon
betweein
the
muscle
and
hook
was
in-
tentionally
kept
short.
This
is
important,
because
the
'available'
length
of
tendon
for
a
muscle
such
as
F.H.L.
is
much
greater
than
for
a
muscle
like
soleus,
and
the
incorporation
of
a
long
length
(-
2
cm)
of
tendon
in
the
recording
system
introduces
an
appreciable
distor-
tion.
The
total
compliance
of
the
recording
system
(short
length
of
tendon,
steel
hook
and
strain
gauge)
was
typically
0
5
mm/kg,
and
the
introduction
of
2
cm
of
tendon
changed
this
figure
to
3
mm/kg.
These
figures
indicate
only
that
part
of
the
total
compliance
over
which
the
experimenter
has
some
control.
It
takes
no
account
of
the
intramuscular
tendon
or
the
series
elastic
elements
within
the
muscle
fibres.
In
order
to
stabilize
the
leg,
steel
twist
drills
were
inserted
into
both
ends
of
the
tibia
and
then
mounted
in
chucks
which
were
magneti-
cally
anchored
to
a
massive
metal
table
upon
which
all
the
associated
equipment
was
also
located.
The
skin
flaps
formed
by
the
primary
incision
were
sewn
back
to
metal
supports,
thus
creating
a
pool
of
some
50-200
ml.
capacity
which
was
filled
with
warmed
liquid
paraffin.
Once
filled,
the
temperature
of
this
paraffin
pool
was
maintained
between
36-5
and
37.50
C
by
means
of
heaters
and
even
temperature
distribution
ensured
by
stirring.
Care
was
taken
to
see
that
the
muscles
were
always
fully
immersed
in
the
paraffin.
On
those
occasions
when
a
tendon
was
out
of
the
pool
during
recording
it
was
covered
with
a
wisp
of
cotton-wool
soaked
in
paraffin.
In
addition
the
cat's
body
temperature
was
continually
monitored
and
the
reading
used
to
regulate
the
current
supplied
to
an
electric
blanket
on
which
the
cat
rested.
The
device
used
was
similar
to
that
described
by
Krnjevic'
&
Mitchell
(1961).
In
order
to
allow
access
for
the
strain
gauge
the
posterior
part
of
the
calcaneum
was
excised
and
the
cat's
foot
fixed
in
maximum
dorsiflexion.
The
master
stimulator
(Digitimer,
Devices
Ltd.)
allowed
the
programming
of
up
to
five
pulses
separated
by
individually
variable
intervals
during
each
oscillograph
sweep.
Each
pulse
could
be
used
to
initiate
a
single
stimulus,
or
any
two
pulses
could
be
used
to
gate
a
tetanic
train
of
pulses,
the
train
starting
synchronously
with
the
first
of
the
two
pulses
and
stopping
at
the
second.
The
frequency
of
the
tetanic
train
was
controlled
by
a
separate
unit,
the
fixed
frequencies
available
ranging
from
1
to
1000
pulses/sec.
The
calibration
accuracy
was
determined
by
a
10
kc
quartz
crystal.
All
pulses
whether
single
or
repetitive
were
fed
to
the
nerve
through
a
transistorized
stimulus
isolation
unit.
For
each
input
pulse
this
unit
provided
an
earth-free
output
pulse
variable
in
duration
and
intensity.
The
output
impe-
dance
of
the
stimulus
isolation
unit
was
500
ohms.
The
stimuli
were
applied
to
the
nerve
through
platinum
or
silver-wire
electrodes.
Recording.
(a)
Mlechanical.
Isometric
tension
was
measured
by
means
of
either
Statham
or
Langham
Thompson
unbonded
wire
strain
gauge
bridges.
The
particular
transducer
used
dependedonthesizeof
the
cat
and
was
either
G
1
64
orG
1
80
(Statham)
or
UF
2
(Langham
Thompson).
These
gauges
had
tension
maxima
of
1-9,
2-4
and
4-8
kg
and
measured
unloaded
natural
frequencies
of
approximately
720,
840
and
1100
c/s
respectively.
All
gauges
were
d.c.
excited
(15-20
V)
and
had
measured
non-linearities
of
less
than
+
1
%
full
scale.
Calibrations
were
obtained
by
shunting
one
of
the
arms
of
the
transducer
bridge
with
precision
resistors
(0-1
%
Alma).
The
accuracy
of
such
calibrations
was
periodically
checked
by
loading
the
various
strain
gauges
with
weights.
The
voltage
output
from
the
strain
gauge
338
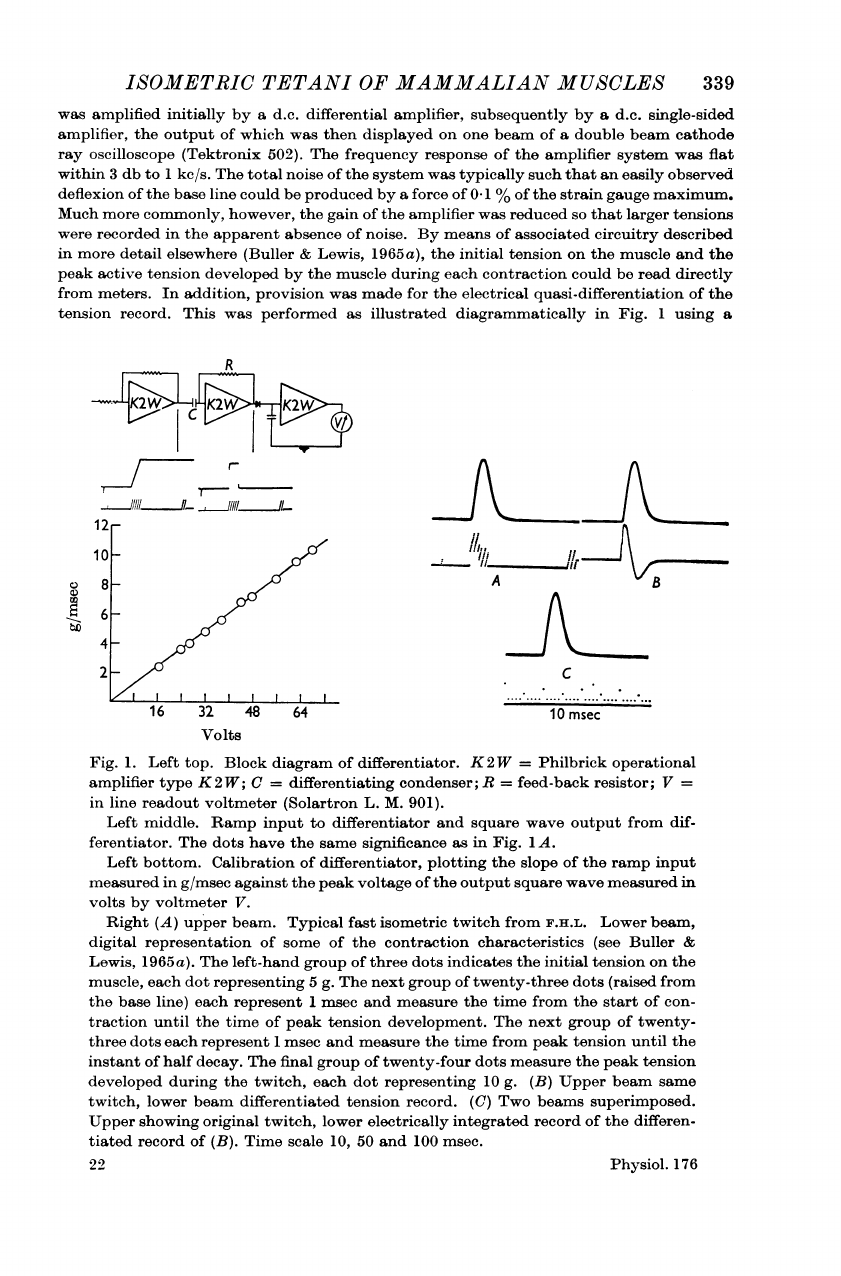
ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
339
was
amplified
initially
by
a
d.c.
differential
amplifier,
subsequently
by
a
d.c.
single-sided
amplifier,
the
output
of
which
was
then
displayed
on
one
beam
of
a
double
beam
cathode
ray
oscilloscope
(Tektronix
502).
The
frequency
response
of
the
amplifier
system
was
flat
within
3
db
to
1
kc/s.
The
total
noise
of
the
system
was
typically
such
that
an
easily
observed
deflexion
of
the
base
line
could
be
produced
by
a
force
of
0-1
%
of
the
strain
gauge
maximum.
Much
more
commonly,
however,
the
gain
of
the
amplifier
was
reduced
so
that
larger
tensions
were
recorded
in
the
apparent
absence
of
noise.
By
means
of
associated
circuitry
described
in
more
detail
elsewhere
(Buller
&
Lewis,
1965a),
the
initial
tension
on
the
muscle
and
the
peak
active
tension
developed
by
the
muscle
during
each
contraction
could
be
read
directly
from
meters.
In
addition,
provision
was
made
for
the
electrical
quasi-differentiation
of
the
tension
record.
This
was
performed
as
illustrated
diagrammatically
in
Fig.
1
using
a
R
12C
10
I
I1
6
-
~
~
~ ~ ~ ~ ~ ~ ~
4-
2
C
16
32
48
64
10
msec
Volts
Fig.
1.
Left
top.
Block
diagram
of
differentiator.
K2W
=
Philbrick
operational
amplifier
type
K
2
W;
C
=
differentiating
condenser;
R
=
feed-back
resistor;
V
=
in
line
readout
voltmeter
(Solartron
L.
M.
901).
Left
middle.
Ramp
input
to
differentiator
and
square
wave
output
from
dif-
ferentiator.
The
dots
have
the
same
significance
as
in
Fig.
1
A.
Left
bottom.
Calibration
of
differentiator,
plotting
the
slope
of
the
ramp
input
measured
in
g/msec
against
the
peak
voltage
of
the
output
square
wave
measured
in
volts
by
voltmeter
V.
Right
(A)
upper
beam.
Typical
fast
isometric
twitch
from
F.H.L.
Lower
beam,
digital
representation
of
some
of
the
contraction
characteristics
(see
Buller
&
Lewis,
1965
a).
The
left-hand
group
of
three
dots
indicates
the
initial
tension
on
the
muscle,
each
dot
representing
5
g.
The
next
group
of
twenty-three
dots
(raised
from
the
base
line)
each
represent
1
msec
and
measure
the
time
from
the
start
of
con-
traction
until
the
time
of
peak
tension
development.
The
next
group
of
twenty-
three
dots
each
represent
1
msec
and
measure
the
time
from
peak
tension
until
the
instant
of
half
decay.
The
final
group
of
twenty-four
dots
measure
the
peak
tension
developed
during
the
twitch,
each
dot
representing
10
g.
(B)
Upper
beam
same
twitch,
lower
beam
differentiated
tension
record.
(C)
Two
beams
superimposed.
Upper
showing
original
twitch,
lower
electrically
integrated
record
of
the
differen-
tiated
record
of
(B).
Time
scale
10,
50
and
100
msec.
22
Physiol.
176

340
A.
J.
BULLER
AND
D.
M.
LEWIS
Philbrick
K2W
operational
amplifier
connected
as
a
differentiator.
Switched
input
capacitors
provided
differentiating
time
constants
of
between
100
tusec
and
1
msec,
while
high-frequency
noise
was
reduced
by
adding
series
resistance to
C
(Fig.
1)
and
shunting
R
with
a
small
capacitance.
The
advantages
of
using
an
operational
amplifier
as
a
differentiator
rather
than
a
simple
input
RC
network
followed
by
a
voltage
amplifier
proved
considerable
(cf.
Korn
&
Korn,
1956).
The
differentiator
was
followed
by
a peak-reading
voltmeter
and
the
peak
voltage
developed
by
the
differentiator
was
displayed
on an
in-line
readout
voltmeter
(Solartron
L.
M.
901).
The
accuracy
of
the
differentiator
was
tested
by
electrically
integra-
ting
the
differentiated
tension
record
(see
Fig.
1).
The
linearity
of
the
peak-reading
device
was
measured
by
feeding
voltage
ramps
equivalent
to
known
rates
of
change
of
input
voltage
per
second
into
the
differentiator
and
measuring
the
square
wave
voltage
developed
by
the
differentiator
(see
Fig.
1).
By
suitable
scaling
the
reading
of
the
in-line
readout
voltmeter
could
be
equated
to
changes
of
tension
(measured
in
g/msec)
at
the
input
of
any
one
of
the
strain
gauges
used.
If
required
the
output
of
the
differentiator
could
be
displayed
on
one
beam
of
the
oscilloscope.
(b)
Electrical.
Action
potentials
from
muscle
were
recorded
either
by
means
of
belly
tendon
leads
of
silver
wire
or
by
means
of
bipolar
concentric
needle
electrodes.
In
either
case
the
signals
were
fed
to
a
conventional
a.c.
coupled
preamplifier
(Tektronix
122)
with
3
db
points
at
80
c/s
and
10
kc/s.
The
output
from
the
preamplifier
was
a.c.
coupled
(O
I,uF
and
1
MQ)
into
a
Tektronix
502
oscilloscope.
Time
traces
were
derived
from
count-down
circuits
driven
from
a
10
kc/s
crystal
oscillator.
Permanent
records
were
obtained
by
photographing
the
face
of
the
cathode
ray
tube
on
film
(Ilford
type
5B62)
using
a
camera
system
with
an
optical
reduction
of
approximately
1:
3.
The
dissection
completed,
the
cat
was
set
up
and
the
pool
formed.
The
animal
was
then
left
for
half
to
one
hour
for
the
muscles
to
come
into
temperature
equilibrium
with
the
paraf-
fin
in
the
pool,
since
despite
efforts
to
the
contrary
the
muscles
often
cooled
during
the
dissection.
After
this
the
motor
nerve
of
the
muscle
to
be
studied
was
stimulated
approximately
once
every
6
or
9
sec
with
shocks
of
30
or
100
gsec
duration
and
2-5
V
intensity
(approximately
3
times
that
necessary
to
produce
a
maximal
contraction).
The
lower
repetition
frequency
was
used
particularly
for
fast
muscles
such
as
flexor
hallucis
longus.
This
reduced
to
a
very
low
level
the
potentiating
effects
of
previous
stimuli
on
the
mechanical
responses.
Considerable
care
was
then
taken
to
align
correctly
the
strain
gauge
in
the
natural
line
of
pull
of
the
muscle,
and
to
apply
that
amount
of
initial
tension
to
the
muscle
which
produced
the
maximal
twitch
response.
The
initial
tension
was
adjusted
by
means
of
a
micrometer
drive
coupled
to
the
strain
gauge.
The
extreme
importance
of
care
at
this
stage
has
been
stressed
by
Buller,
Eccles
&
Eccles
(1960a)
and
in
more
detailed
studies
by
Buller
&
Lewis
(1963
b).
Next,
evidence
was
sought
for
any
effect
on
the
muscle
twitch
of
a
back
response
in
the
motor
nerve
fibres
(Brown
&
Matthews,
1960;
Buller
&
Lewis,
1963b).
This
was
done
by
applying
two
maximal
stimuli
to
the
motor
nerve
separated
by
intervals
ranging
between
0
5
and
1*5
msec
and
noting
whether
any
decrease
in
the
size
of
the
mechanical
response
occurred.
If
such
an
effect
was
observed
(it
appeared
more
commonly
in
large
cats
and
in
soleus)
the
muscle
was
excluded
from
the
present
study.
Measurements
were
then
commenced
of
either
the
effects
of
two
stimuli
at
various
intervals
or
the
effects
of
short
tetani
(100-400
msec)
at
various
frequencies.
After
a
tetanus
the
motor
nerve
was
stimulated
by
single
shocks
once
every
9
sec
until
any
alteration
which
had
taken
place
in
the
twitch
size
(post-tetanic
potentiation,
Brown
&
von
Euler,
1938)
and/or
rate
of
tension
development
had
disappeared.
This
rarely
took
longer
than
60
sec
and
was
easily
assessed
since
both
these
contraction
characteristics
could
be
directly
read
out
on
meters
after
each
twitch.
Usually
the
frequency
of
the
tetanic
stimuli
was
increased
in
steps
up
to
a
maximuim
of
400-600pulses/sec
and
in
afew
cases
to
1000
pulses/sec
and
then
reduced
through
intermediate
frequencies
to
ensure
that
no
changes
had
taken
place
in
the
muscle
as
a
result
of
the
high-
ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
341
frequency
activation.
A
similar
procedure
was
adopted
with
the
two-stimuli
experiments.
Occasionally
the
frequency
changes
or
intervals
were
randomized.
No
differences
were
noted
in
the
results
obtained.
At
the
conclusion
of
the
experiment
the
muscles
used
were
dissected
out
of
the
body,
blotted
'dry'
with
filter
paper
and
weighed.
The
over-all
length
of
the
muscle
(but
excluding
any
tendon)
was
also
measured.
Source8
of
error.
The
finite
time
constant
of
the
differentiating
network
and
the
peak
voltage
condenser
(Fig.
1
block
diagram)
introduce
errors
in
the
reading
of
the
maximum
rate
of
change
of
tension.
However,
calculation
shows
that
the
maxrimum
error
produced
with
the
highest
rates
of
changeencountered
was
of
theorderof
-5
%.
Amore
important
sourceof
error
was
the
occasional
deterioration
of
the
muscles
following
repeated
tetani.
The
changes
ob-
served
were
effectively
confined
to
the
fast
muscles
studied
and
were
evidenced
as
a
reduced
peak
tension
and
decreased
maximuim
rate
of
tension
rise
during
the
twitch
while
the
peak
te-
tanic
tension
and
the
rate
of
risewerelittleaffected.
Wehavenotinvestigatedthisphenomenon
in
detail,
but
have
rejected
results
in
which
the
peak
twitch
tension
fell
by
15
%
or
more
during
the
course
of
the
whole
experiment.
Apart
from
these
occasional
exceptions
the
muscles
remained
in
excellent
condition,
and
consistent
responses
could
be
obtained
over
many
hours.
Soleus
F.H.L
83
12-5
32
50
25
50
80
125
100
msec
10
msec
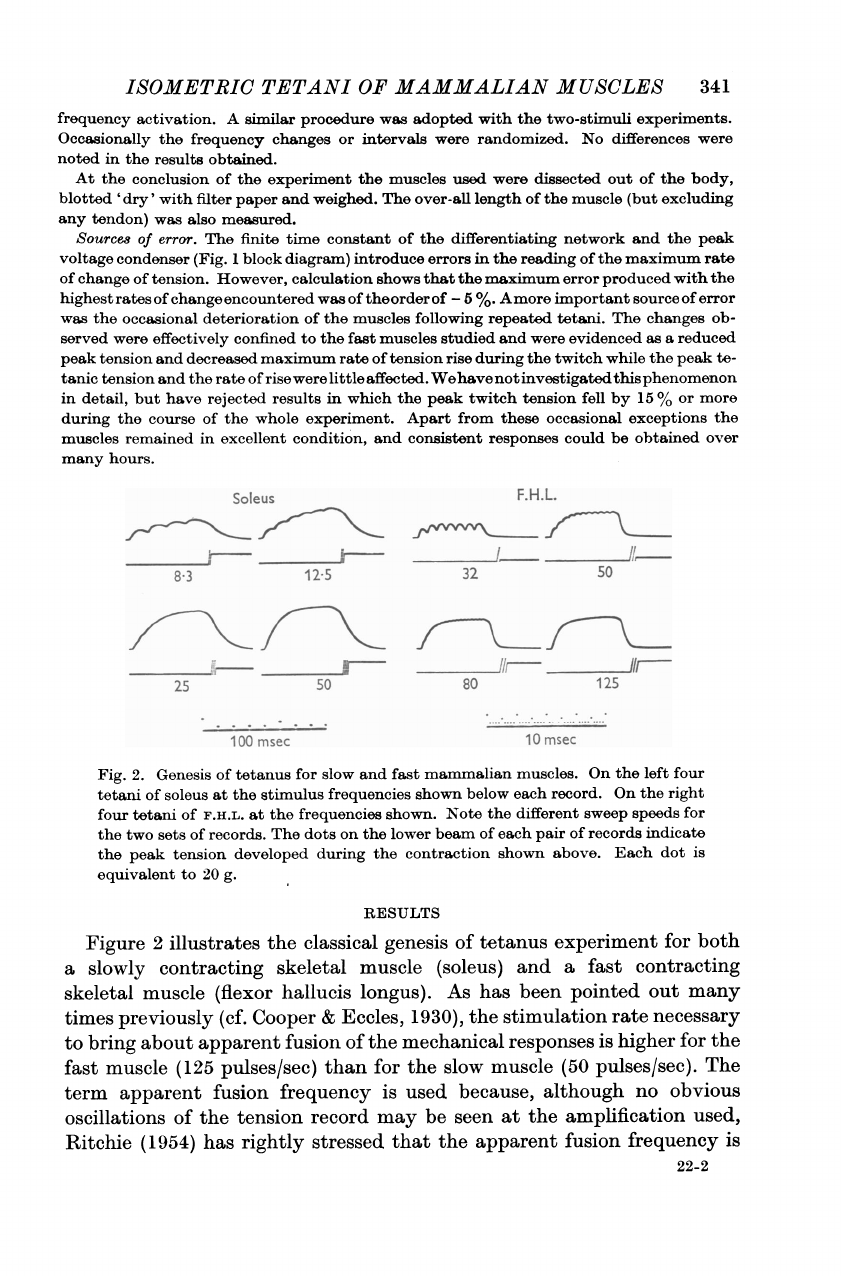
Fig.
2.
Genesis
of
tetanus
for
slow
and
fast
mammalian
muscles.
On
the
left
four
tetani
of
soleus
at
the
stimulus
frequencies
shown
below
each
record.
On
the
right
four
tetani
of
F.H.L.
at
the
frequencies
shown.
Note
the
different
sweep
speeds
for
the
two
sets
of
records.
The
dots
on
the
lower
beam
of
each
pair
of
records
indicate
the
peak
tension
developed
during
the
contraction
shown
above.
Each
dot
is
equivalent
to
20
g.
RESULTS
Figure
2
illustrates
the
classical
genesis
of
tetanus
experiment
for
both
a
slowly
contracting
skeletal
muscle
(soleus)
and
a
fast
contracting
skeletal
muscle
(flexor
hallucis
longus).
As
has
been
pointed
out
many
times
previously
(cf.
Cooper
&
Eccles,
1930),
the
stimulation
rate
necessary
to
bring
about
apparent
fusion
of
the
mechanical
responses
is
higher
for
the
fast
muscle
(125
pulses/sec)
than
for
the
slow
muscle
(50
pulses/sec).
The
term
apparent
fusion
frequency
is
used
because,
although
no
obvious
oscillations
of
the
tension
record
may
be
seen
at
the
amplification
used,
Ritchie
(1954)
has
rightly
stressed
that
the
apparent
fusion
frequency
is
22-2

A.
J.
BULLER
AND
D.
M.
LEWIS
dependent
on
the
sensitivity
of
the
recording
system.
If
the
apparently
fused
tetani
of
Fig.
2
were
examined
at
higher
amplification,
oscillations
at
the
stimulus
frequency
would
certainly
be
visible.
However,
from
the
maximum
frequencies
illustrated
(50
pulses/sec
for
soleus
and
125
pulses/
sec
for
F.H.L.)
any
further
increase
in
frequency
produces
negligible
change
in
the
peak
tension
developed
by
the
muscle.
Definition
must
here
be
made
of
peak
tension
since,
while
the
fast
muscles
usually
show
a
plateau
of
tension,
the
slow
muscles
often
show
a
slow
rise
of
tension
for
several
hundred
milliseconds
after
the
initial
faster
rise.
Peak
tension
will
there-
fore
be
defined
for
the
purposes
of
this
paper
as
the
maximum
tension
reached
400
msec
after
the
first
stimulus
of
a
tetanic
train,
since
by
this
time
any
residual
climb
in
tension
is
small.
While
it
is
true
that
no
increase
occurs
in
the
peak
tension
developed
if
the
stimulation
frequency
exceeds
50
pulses/sec
for
soleus
and
125
pulses/
sec
for
F.H.L.
it
is
apparent
from
Fig.
3
that
the
rate
at
which
the
peak
tension
is
developed
is
considerably
enhanced
by
further
increases
in
the
stimulation
frequency.
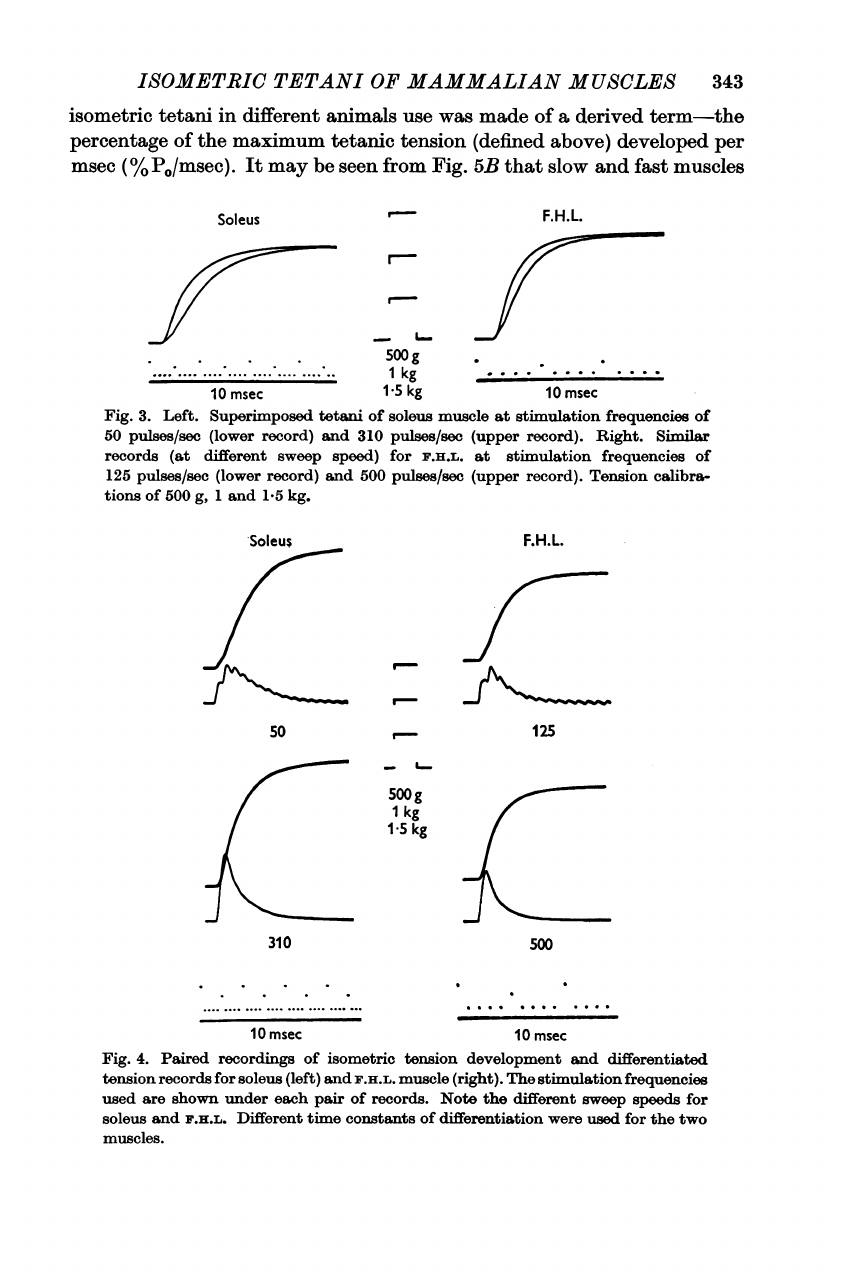
Figure
3
shows
superimposed
isometric
tension
records
of
two
tetani
of
soleus
at
stimulation
rates
of
50
pulses/sec
and
310
pulses/sec
and
two
tetani
of
F.H.L.
at
stimulation
rates
of
125
pulses/
sec
and
500
pulses/sec.
In
order
to
study
this
change
in
the
rate
of
tension
development
more
closely
differentiated
records
of
the
tension
rise
were
employed
(see
Methods)
and
an
illustration
of
such
records
obtained
during
tetanic
stimu-
lation
are
shown
in
the
lower
beam
recordings
of
Fig.
4.
The
differentiated
tension
records
serve
in
fact
to
increase
the
effective
amplification
of
the
recording
system
and
clearly
demonstrate
changes
in
the
rate
of
tension
build
up
with
time.
In
the
lower
oscillographic
records
of
both
the
soleus
tetanus
at
50
pulses/sec
and
the
flexor
hallucis
longus
tetanus
at
125
pulses/
sec
obvious
oscillations
are
apparent
while
with
the
tetani
at
310
pulses/
sec
and
500
pulses/sec
the
differentiated
records
are
much
smoother.
Close
examination
of
Fig.
4
will
also
show
that
in
both
the
slow
and
fast
muscle
the
maximum
rate
of
change
of
tension
(the
peak
of
the
differential
record)
is
greater
and
occurs
earlier
with
the
higher
frequency
of
stimula-
tion.
This
latter
point
is
illustrated
in
Fig.
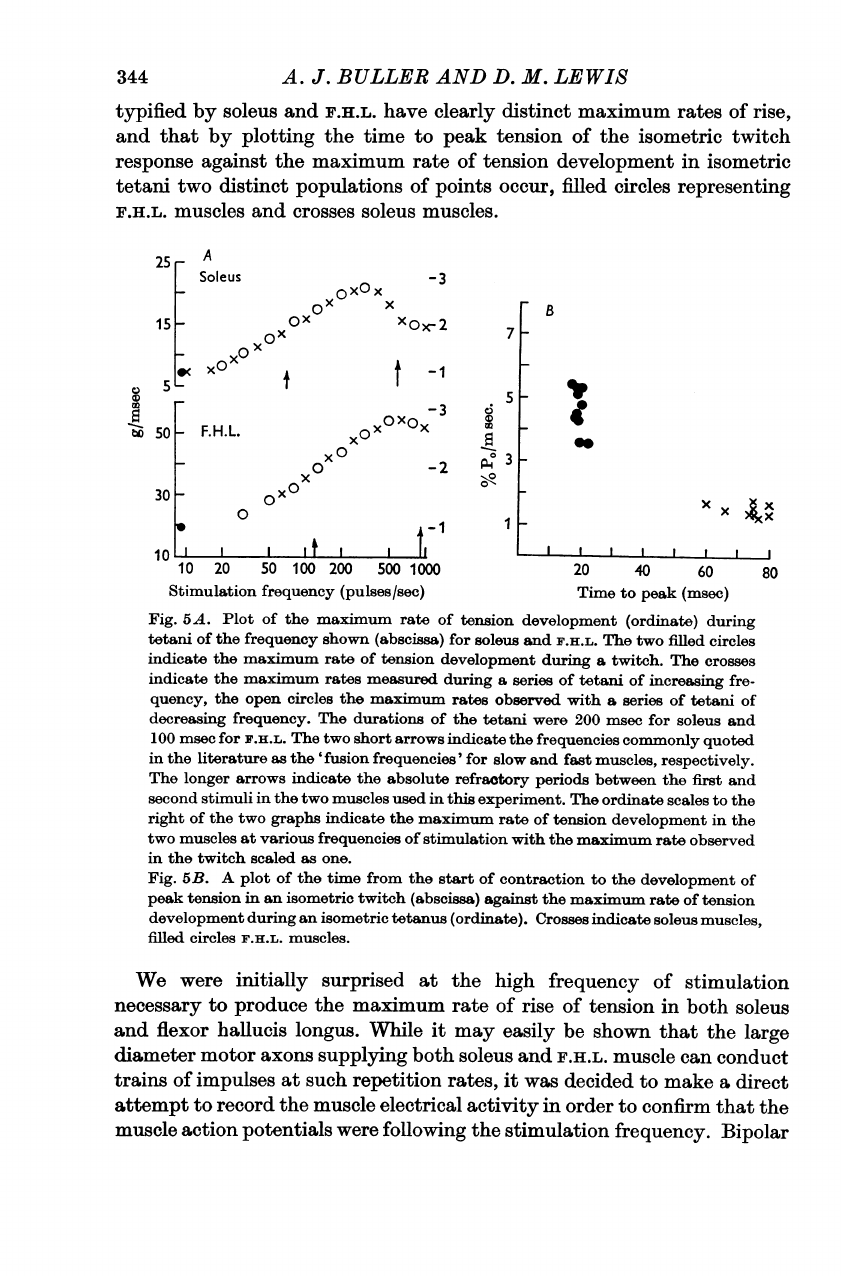
5A,
where
the
maximum
rate
of
change
of
tension
measured
in
g/msec
is
plotted
against
stimulus
frequency.
The
filled
circles
show
the
maximum
rate
of
rise
of
tension
during
a
single
twitch,
the
crosses
show
points
observed
with
increasing
stimulus
fre-
quency
and
the
open
circles
points
obtained
with
decreasing
stimulus
frequency.
For
any
particular
muscle
the
shape
of
the
curve
is
extremely
reproducible
from
animal
to
animal,
though
the
absolute
value
in
g/msec
varies.
In
order
to
compare
the
maximum
rate
of
tension
development
during
342
ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
343
isometric
tetani
in
different
animals
use
was
made
of
a
derived
term-the
percentage
of
the
maximum
tetanic
tension
(defined
above)
developed
per
msec
(%
P0/msec).
It
may
be
seen
from
Fig.
5B
that
slow
and
fast
muscles
Soleus
F.H.L.
500g
.......
.........
.........
.......
I
kg
.
..
..
.. ..
..
.
10
msec
1-5
kg
10
msec
Fig.
3.
Left.
Superimposed
tetani
of
soleus
muscle
at
stimulation
frequencies
of
50
pulses/sec
(lower
record)
and
310
pulses/sec
(upper
record).
Right.
Similar
records
(at
different
sweep
speed)
for
F.H.L.
at
stimulation
frequencies
of
125
pulses/sec
(lower
record)
and
500
pulses/sec
(upper
record).
Tension
calibra-
tions
of
500
g,
1
and
1-5
kg.
-Soleus
50
310
F.H.L.
125
_
500
g
1
kg
1-5
kg
500
..................
....
....
....
....
....
....
.........
.
.
.
.
. .
.
.
.
.
.
.
..
10
msec
10
msec
Fig.
4.
Paired
recordings
of
isometric
tension
development
and
differentiated
tension
records
for
soleus
(left)
and
F.H.L.
muscle
(right).
The
stimulation
frequencies
used
are
shown
under
each
pair
of
records.
Note
the
different
sweep
speeds
for
soleus
and
F.H.L.
Different
time
constants
of
differentiation
were
used
for
the
two
muscles.
344
A.
J.
BULLER
AND
D.
M.
LEWIS
typified
by
soleus
and
F.H.L.
have
clearly
distinct
maximum
rates
of
rise,
and
that
by
plotting
the
time
to
peak
tension
of
the
isometric
twitch
response
against
the
maximum
rate
of
tension
development
in
isometric
tetani
two
distinct
populations
of
points
occur,
filled
circles
representing
F.H.L.
muscles
and
crosses
soleus
muscles.
25
A
Soleus
-3
I
~~~~0x
15
-
Ox
x
Ox
2
E
-1
5
-e
10
30
t
-2
10~)
20
50
100
200
20
Stimulation
frequency
(pulses/see)
Time
to
peak
(msec)
Fig.
5A.
Plot
of
the
maxrimum
rate
of
tension
development
(ordinate)
during
tetani
of
the
frequency
shown
(abscissa)
for
soleus
anld
F.H.L.
The
two
filled
circles
indicate
the
maxrimum
rate
of
tension
development
during
a
twitch.
The
crosses
indicate
the
maximum
rates
measured
during
a
series
of
tetani
of
increasing
fre-
quency,
the
open
circles
the
maximulm
rates
observed
with
a
series
of
tetani
of
decreasing
frequency.
The
durations
of
the
tetani
were
200
msec
for
soleus
and
100
msec
for
F.H.L.
The
two
short
arrows
indicate
the
frequencies
commonly
quoted
in
the
literature
as
the
'fusion
frequencies'
for
slow
and
fast
muscles,
respectively.
The
longer
arrows
indicate
the
absolute
refractory
periods
between
the
first
and
second
stimuli
in
the
two
muscles
used
in
this
exrperiment.
The
ordinate
scales
to
the
right
of
the
two
graphs
indicate
the
maxrimum
rate
of
tension
development
in
the
two
muscles
at
various
frequencies
of
stimulation
with
the
maximum
rate
observed
in
the
twitch
scaled
as
one.
Fig.
5B.
A
plot
of
the
time
from
the
start
of
contraction
to
the
development
of
peak
tension
in
an
isometric
twitch
(abscissa)
against
the
maximum
rate
of
tension
development
during
an
isometric
tetanus
(ordinate).
Crosses
indicate
soleus
muscles,
filled
circles
F.H.L.
muscles.
We
were
initially
surprised
at
the
high
frequency
of
stimulation
necessary
to
produce
the
maxsimulm
rate
of
rise
of
tension
in
both
soleus
and
flexor
hallucis
longus.
While
it
may
easily
be
shown
that
the
large
diameter
motor
axonLs
supplying
both
soleus
and
F.H.L.
muscle
can
conduct
trains
of
impulses
at
such
repetition
rates,
it
was
decided
to
make
a
direct
attempt
to
record
the
muscle
electrical
activity
in
order
to
confirm
that
the
muscle
action
potentials
were
following
the
stimulation
frequency.
Bipolar
ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
345
concentric
needle
electrodes
were
used,
but
because
of
the
relatively
long
biphasic
muscle
action
potentials
it is
often
difficult
to
interpret
the
records
with
the
highest
stimulation
frequencies.
Movement
of
the
muscle
during
the
contraction
also
served
to
alter
the
recording
conditions
at
the
elec-
trode
tip,
and
to
make
difficult
the
interpretation
of
changes
in
amplitude
of
the
action
potentials.
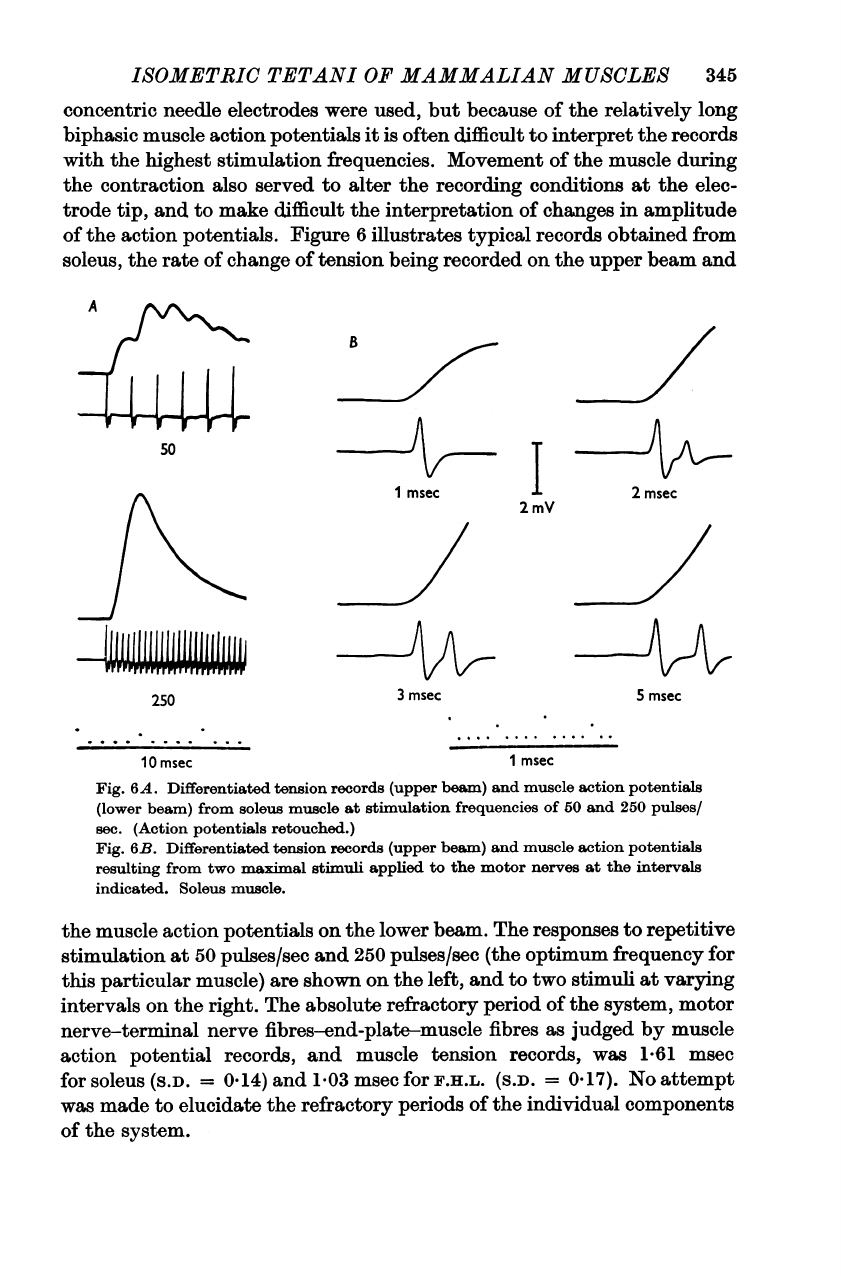
Figure
6
illustrates
typical
records
obtained
from
soleus,
the
rate
of
change
of
tension
being
recorded
on
the
upper
beam
and
50
1
1
msec
2
msec
2
mV
250
3
msec
5
msec
..
...
...
.....
10
msec
1
msec
Fig.
6A.
Differentiated
tension
records
(upper
beam)
and
muscle
action
potentials
(lower
beam)
from
soleus
muscle
at
stimulation
frequencies
of
50
and
250
pulses/
sec.
(Action
potentials
retouched.)
Fig.
6B.
Differentiated
tension
records
(upper
beam)
and
muscle
action
potentials
resulting
from
two
maximal
stimuli
applied
to
the
motor
nerves
at
the
intervals
indicated.
Soleus
muscle.
the
muscle
action
potentials
on
the
lower
beam.
The
responses
to
repetitive
stimulation
at
50
pulses/sec
and
250
pulses/sec
(the
optimum
frequency
for
this
particular
muscle)
are
shown
on
the
left,
and
to
two
stimuli
at
varying
intervals
on
the
right.
The
absolute
refractory
period
of
the
system,
motor
nerve-terminal
nerve
fibres-end-plate-muscle
fibres
as
judged
by
muscle
action
potential
records,
and
muscle
tension
records,
was
1-61
msec
for
soleus
(S.D.
=
0.14)
and
1
03
msec
for
F.H.L.
(S.D.
=
0-17).
No
attempt
was
made
to
elucidate
the
refractory
periods
of
the
individual
components
of
the
system.
346
A.
J.
BULLER
AND
D.
M.
LEWIS
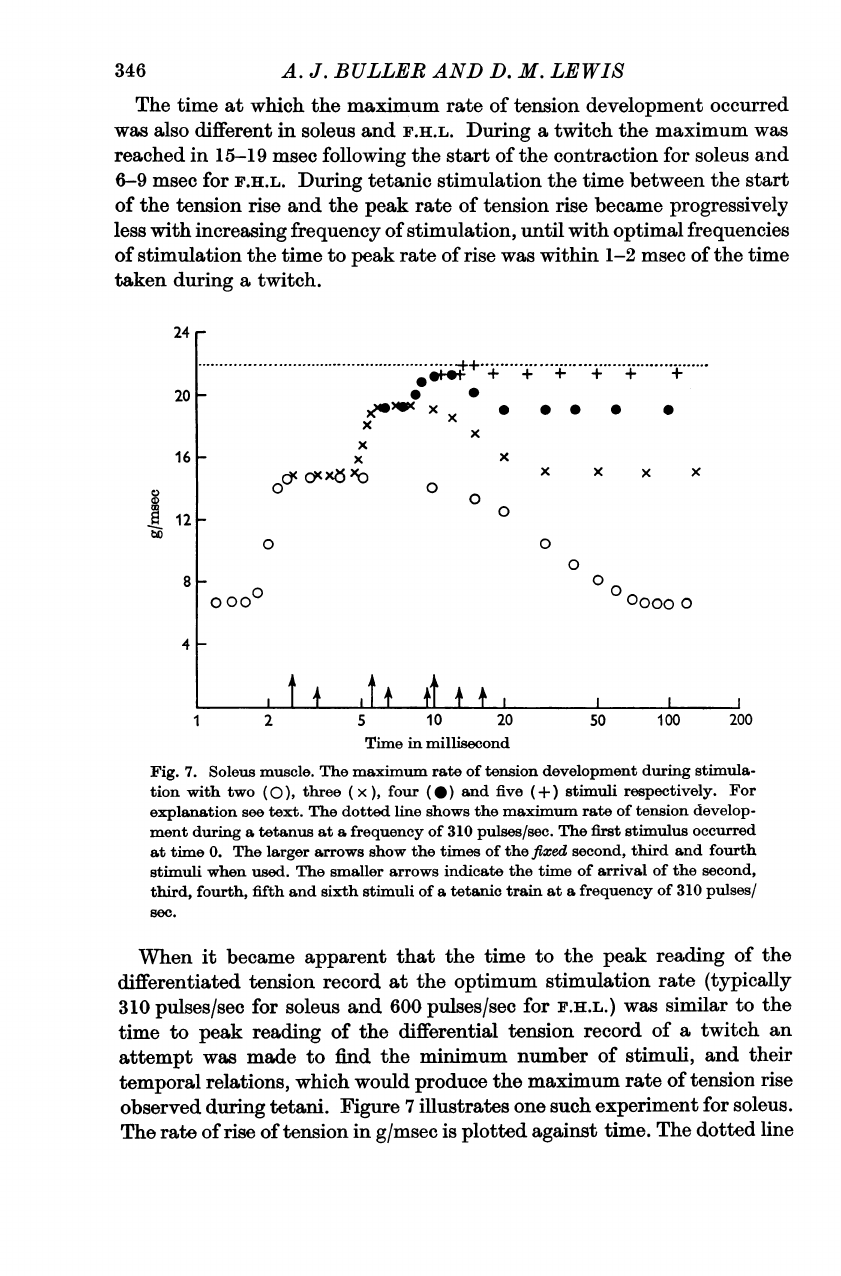
The
time
at
which
the
maximum
rate
of
tension
development
occurred
was
also
different
in
soleus
and
F.H.L.
During
a
twitch
the
maximum
was
reached
in
15-19
msec
following
the
start
of
the
contraction
for
soleus
and
6-9
msec
for
F.H.L.
During
tetanic
stimulation
the
time
between
the
start
of
the
tension
rise
and
the
peak
rate
of
tension
rise
became
progressively
less
with
increasing
frequency
of
stimulation,
until
with
optimal
frequencies
of
stimulation
the
time
to
peak
rate
of
rise
was
within
1-2
msec
of
the
time
taken
during
a
twitch.
24_
*
+
+
+
+
+
.+.
20
-
0
0
x
x
K
16
x
x
C)~~~~cca
dC$X?j
x
x
x
x
x
0
~~~~0
0
12
0
bo
0
0
0
8
0
00
0
0
0000~
0
4
,
t4
+
+
44
+,
.
.
1
2
5
10
20
50
100
200
Time
in
millisecond
Fig.
7.
Soleus
muscle.
The
maximum
rate
of
tension
development
during
stimula-
tion
with
two
(0),
three
(x),
four
(0)
and
five
(+)
stimuli
respectively.
For
explanation
see
text.
The
dotted
line
shows
the
maximujm
rate
of
tension
develop-
ment
during
a
tetanus
at
a
frequency
of
310
pulses/sec.
The
first
stimulus
occurred
at
time
0.
The
larger
arrows
show
the
times
of
the
fixed
second,
third
and
fourth
stimuli
when
used.
The
smaller
arrows
indicate
the
time
of
arrival
of
the
second,
third,
fourth,
fifth
and
sixth
stimuli
of
a
tetanic
train
at
a
frequency
of
310
pulses/
Sec.
When
it
became
apparent
that
the
time
to
the
peak
reading
of
the
differentiated
tension
record
at
the
optimum
stimulation
rate
(typically
310
pulses/sec
for
soleus
and
600
pulses/sec
for
F.H.L.)
was
similar
to
the
time
to
peak
reading
of
the
differential
tension
record
of
a
twitch
an
attempt
was
made
to
find
the
minimum
number
of
stimuli,
and
their
temporal
relations,
which
would
produce
the
maximum
rate
of
tension
rise
observed
during
tetani.
Figure
7
illustrates
one
such
experiment
for
soleus.
The
rate
of
rise
of
tension
in
g/msec
is
plotted
against
time.
The
dotted
line
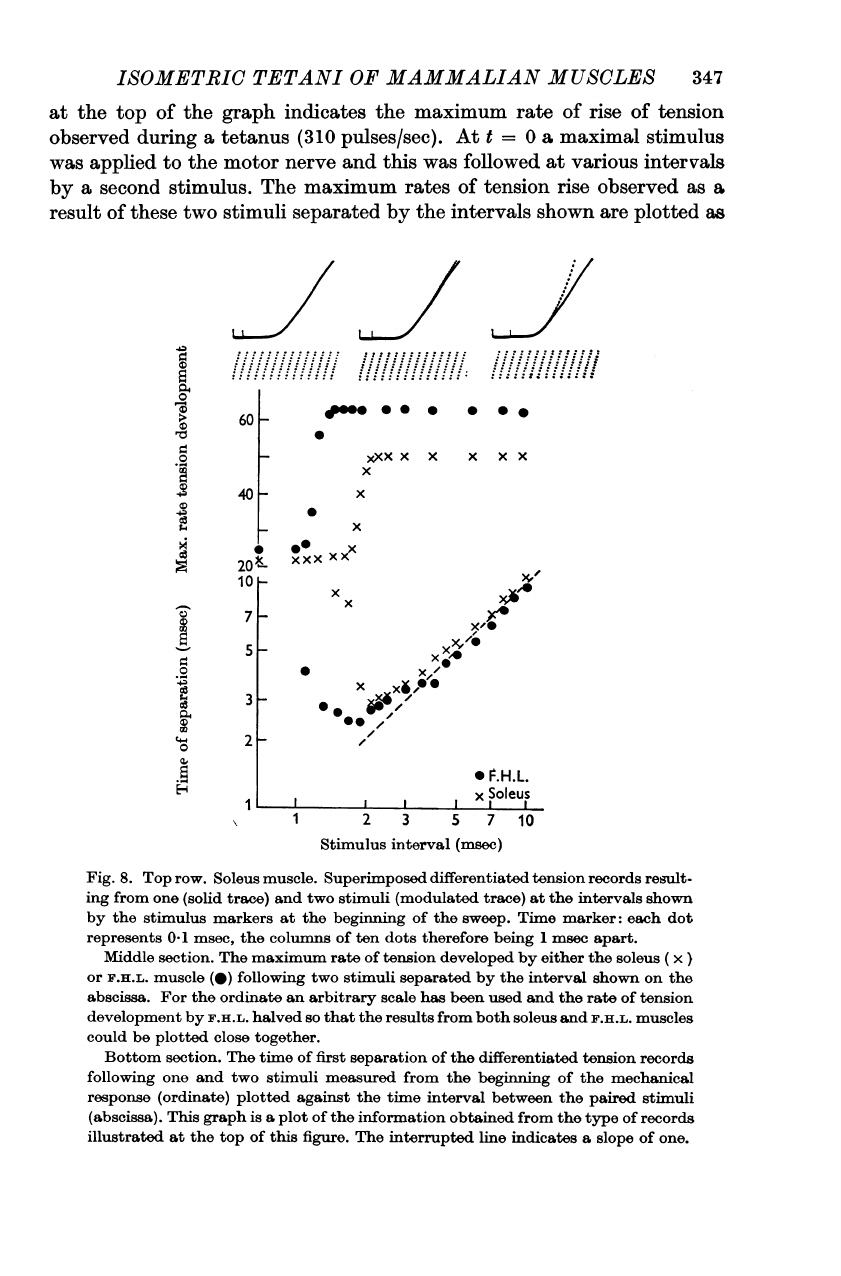
ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
347
at
the
top
of
the
graph
indicates
the
maximum
rate
of
rise
of
tension
observed
during
a
tetanus
(310
pulses/sec).
At
t
=
0
a
maximal
stimulus
was
applied
to
the
motor
nerve
and
this
was
followed
at
various
intervals
by
a
second
stimulus.
The
maximum
rates
of
tension
rise
observed
as
a
result
of
these
two
stimuli
separated
by
the
intervals
shown
are
plotted
as
o
d~~~~~
xXX
X
X
X(
X
X
04
40_
x
0
604
4
0
-
x
20
*
-H.L
10~os
XXX
X
Ev~~~~~
x
Solu
77
X/S
5
>e/S
.0
X~~
3
-4-
2
0
E-i
~~~~~~~~~x
Soleus
1
2
3
5
7
10
Stimulus
interval
(msec)
Fig.
8.
Top
row.
Soleus
muscle.
Superimposed
differentiated
tension
records
result-
ing
from
one
(solid
trace)
and
two
stimuli
(modulated
trace)
at
the
intervals
shown
by
the
stimulus
markers
at
the
beginning
of
the
sweep.
Time
marker:
each
dot
represents
0*1
msec,
the
columns
of
ten
dots
therefore
being
1
msec
apart.
Middle
section.
The
maximum
rate
of
tension
developed
by
either
the
soleus
(
x)
or
F.H.L.
muscle
(0)
following
two
stimuli
separated
by
the
interval
shown
on
the
abscissa.
For
the
ordinate
an
arbitrary
scale
has
been
used
and
the
rate
of
tension
development
by
F.H.L.
halved
so
that
the
results
from
both
soleus
and
F.H.L.
muscles
could
be
plotted
close
together.
Bottom
section.
The
time
of
first
separation
of
the
differentiated
tension
records
following
one
and
two
stimuli
measured
from
the
beginning
of
the
mechanical
response
(ordinate)
plotted
against
the
time
interval
between
the
paired
stimuli
(abscissa).
This
graph
is
a
plot
of
the
information
obtained
from
the
type
of
records
illustrated
at
the
top
of
this
figure.
The
interrupted
line
indicates
a
slope
of
one.

A.
J.
BULLER
AND
D.
M.
LEWIS
open
circles.
It
is
seen
that
following
the
absolute
refractory
period
of
the
total
system
(nerve-end-plate-muscle)
there
is
a
rapid
increase
in
the
maximum
rate
of
tension
rise
as
the
interval
between
the
two
stimuli
is
lengthened.
This
is
followed
by
a
plateau
of
some
3
msec
and
then
a
slow
decline.
Having
plotted
the
curve
of
open
circles
two
stimuli
were
applied
to
the
nerve
with
a
fixed
interval
of
2*5
msec.
A
third
stimulus
was
then
added
at
varying
intervals
after
the
second
of
the
two
fixed
stimuli.
The
maximum
rates
of
rise
of
tension
in
the
contraction
caused
by
the
three
stimuli
are
plotted
in
Fig.
7
as
crosses,
the
time
scale
indicating
the
time
of
third
stimulus
(the
first
two
occurring
at
times
0
and
2-5
msec).
A
similar
but
smaller
rise
in
the
rate
of
tension
development
is
apparent.
This
is
followed
by
a
comparable
plateau
and
decline.
The
filled
circles
indicate
the
responses
to
three
fixed
stimuli
at
times
0,
2-5
and
5-5
msec
and
a
fourth
stimulus
at
the
interval
shown
on
the
abscissa.
The
+
symbols
indicate
the
responses
to
four
fixed
stimuli
at
times
0,
2-5,
5-5
and
10
msec
and
to
a
fifth
stimulus
at
the
interval
shown.
It
may
be
seen
that
in
this
example
five
stimuli
optimally
timed
were
adequate
to
reach
the
maximum
rate
of
tension
development
observed
in
a
tetanus.
Increasing
the
number
of
stimuli
used
above
five
did
not
increase
the
maximum
rate
of
rise
of
tension.
In
other
experiments
the
maximum
rate
of
tension
development
was
reached
with
three
to
six
stimuli,
and
similar
results
were
obtained
with
F.H.L.
Since
it
was
apparent
from
experiments
of
the
type
illustrated
in
Fig.
7
that
in
soleus
two
stimuli
separated
by
only
2*5
msec
were
having
a
marked
influence
on
the
rate
of
rise
of
tension
during
the
mechanical
response
of
the
muscle
it
seemed
pertinent
to
examine
in
more
detail
the
time
of
separation
of
the
mechanical
responses
resulting
from
one
and
two
stimuli.
As
has
been
shown
by
Macpherson
&
Wilkie
(1954),
and
stressed
earlier
in
this
paper,
differentiation
of
the
tension
record
increases
the
resolution
by
effectively
increasing
the
amplification
of
the
recording
system.
The
upper
part
of
Fig.
8
illustrates
superimposed
traces
of
the
differentiated
isometric
tension
records
of
a
soleus
muscle
following
one
and
two
stimuli
applied
at
the
intervals
shown
by
the
added
markers
on
the
upper
beam.
The
oscilloscope
sweep
containing
the
response
to
two
stimuli
has
been
intensity
modulated
(and
therefore
appears
dotted)
in
order
to
distinguish
it.
The
lower
part
of
Fig.
8
illustrates
the
results
obtained
from
one
such
experiment
on
each
of
soleus
(crosses)
and
F.H.L.
(filled
circles)
muscles.
The
interval
between
the
two
stimuli
(abscissa)
is
plotted
against
the
time
after
the
start
of
contraction
at
which
the
two
differentiated
mechanical
records
separate.
The
two
graphs
have
a
similar
shape,
but
because
of
the
longer
absolute
refractory
period
of
soleus
muscle
the
left
limb
of
its
graph
348

ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
349
is
displaced
approximately
1
msec
to
the
right
compared
with
that
of
F.H.L.
The
minimum
time
of
separation
is
also
increased
by
approximately
0
5
msec.
For
both
soleus
and
F.H.L.
muscles,
the
slope
of
the
relation
between
stimulus
interval
and
the
time
of
separation
of
the
mechanical
responses
approximated
to
the
expected
value
of
one
provided
the
stimulus
interval
exceeded
4
msec.
Finally,
it
is
pertinent
to
state
that
we
have
attempted
to
reproduce
all
the
results
described
above
on
curarized
muscle.
However,
in
vivo
we
have
so
far
found
it
impossible
to
stimulate
directly
such
large
muscles
as
soleus
and
F.H.L.
and
obtain
on
increasing
the
stimulus
strength
an
unequivocal
response
equivalent
to
that
obtained
with
a
maximal
motor
nerve
volley.
It
is
certainly
possible
to
stimulate
directly
through
electrodes
between
the
origin
and
tendon
of
the
muscle
and
arbitrarily
adjust
the
stimulus
strength
so
that
the
tension
developed
by
the
muscle
is
similar
to
the
tension
obtained
after
a
maximal
stimulus
to
the
nerve
before
cura-
rization.
However,
in
our
experience
such
stimuli,
even
if
of
short
dura-
tion
(<
1
msec),
cause
some
muscle
fibres
to
discharge
repetitively.
Our
direct
stimulation
experiments
have
therefore
been
confined
to
levels
of
submaximal
stimulation
at
which
repetitive
firing
of
muscle
fibres
is
negligible.
With
a
single
stimulus
of
such
intensity
some
muscle
fibres
which
do
not
respond
by
contracting
are
subliminally
stimulated
and
if
a
second
stimulus
is
applled
sufficiently
rapidly
(<
2
msec)
these
fibres
will
be
recruited
to
the
second
mechanical
response.
However,
we
have
found
this
effect
interferes
very
little
at
tetanic
stimulation
frequencies
below
about
500
pulses/sec.
Under
these
conditions
we
have
confirmed
that
the
maximum
rates
of
tension
rise
during
isometric
contractions
in
curarized
fast
and
slow
muscle
remain
different
and
the
values
obtained
are
similar
to
those
obtained
using
indirect
stimulation.
DISCUSSION
The
experiments
described
above
quantify
the
differences
which
exist
between
the
maximum
rate
of
rise
of
tension
during
isometric
tetani
of
mammalian
fast
and
slow
skeletal
muscles.
While
for
many
years
it
has
been
apparent
that
the
rise
of
tension
during
an
isometric
'fused'
tetanus
of
fast
muscle
is
more
rapid
than
the
rise
of
tension
during
a
tetanus
of
slow
muscle,
the
marked
influence
of
the
rate
of
stimulation
(see
Fig.
5A)
on
both
types
of
muscle
has
not
been
appreciated.
It
is
apparent
that
if
quantitative
comparisons
are
to
be
made
between
normal
muscles
and
muscles
which
have
been
cross-innervated
(Buller
et
al.
1960b)
careful
attention
must
be
paid
to
this
parameter.
These
results
also
serve
to

A.
J.
BULLER
AND
D.
M.
LEWIS
emphasize
the
care
which
must
be
exercised
in
the
use
of
the
term
'fusion
frequency'.
If
used
to
define
the
frequency
at
which
the
individual
con-
tractions
produced
by
the
successive
stimuli
of
a
tetanic
train
can
no
longer
be
identified,
the
term
is
as
much
a
measure
of
the
sensitivity
of
the
recording
system
as
of
the
muscle's
performance
(Ritchie,
1954).
While
it
is
often
desirable
to
compare
the
'apparent
fusion'
frequencies
of
two
muscles
this
should
only
be
done
after
due
consideration
of
the
recording
conditions
for
each
muscle.
That
the
results
described
for
the
two
types
of
mammalian
skeletal
muscle
are
due
to
differences
of
the
contractile
mechanisms
and
not
to
differences
of
the
motor-nerve
fibres
or
end-plates
may
be
inferred
from
the
fact
that
similar
results
were
obtained
by
direct
stimulation
of
cura-
rized
muscles.
In
addition,
Professor
Sandow
has
informed
us
that
he
has
now
observed
a
comparable
effect
of
tetanic
stimulation
frequency
on
the
rate
of
rise
of
isometric
tension
in
frog
sartorius
muscles
using
'all
over'
(massive)
stimulation
(cf.
Mostofsky
&
Sandow,
1951).
From
our
results
with
tetanic
stimulation
it is
impossible
to
decide
whether
the
maximum
rate
of
tension
development
of
which
the
contractile
machinery
is
ultimately
capable
is
ever
reached.
In
Fig.
5A,
which
was
obtained
with
indirect
stimulation,
there
is
no
plateau
to
either
curve,
and
the
fall
off
in
the
maximum
rate
of
tension
development
with
the
highest
stimulation
frequencies
corresponds
very
precisely
with
what
is
to
be
expected
from
the
total
refractory
periods
of
the
two
systems.
In
con-
sidering
the
limitations
imposed
by
the
refractory
period
due
allowance
must
be
made
for
the
lengthening
of
the
absolute
refractory
period
which
occurs
with
successive
stimuli.
This
is
apparent
in
Fig.
7,
where
the
absolute
refractory
period
of
the
system
was
17
msec
following
the
first
stimulus,
2*5
msec
following
the
second
stimulus
and
3-4
msec
following
the
third
stimulus.
With
direct
stimulation
using
submaximal
stimuli
as
described
under
Methods,
recruitment
of
additional
muscle
fibres
occurs
if
the
separa-
tion
between
successive
stimuli
becomes
very
short
(<
2
msec)
and
there-
fore
the
results
obtained
with
the
highest
tetanic
frequencies
are
again
ambiguous.
In
either
case,
however,
the
steady
increase
observed
in
the
maximum
rate
of
tension
development
with
increasing
stimulation
fre-
quency
suggests
that
if
a
plateau
of
active
state
(H-ill,
1949)
occurs
in
the
sarcomeres
of
these
muscles
following
each
stimulus
its
duration
cannot
exceed
approximately
3
msec
in
soleus
or
2
msec
in
F.H.L.
The
results
from
the
two
stimulus
experiments
are
in
complete
accord
with
this
view.
As
explained
for
the
frog
sartorius
muscle
at
00
C
by
Macpherson
&
Wilkie
(1954),
if
a
plateau
of
active
state
occurs
following
a
single
stimulus
then,
until
the
end
of
that
plateau,
the
muscle
will
behave
indistinguishably
whether
or
not
a
second
stimulus
is
applied.
By
noting
350

ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
351
the
earliest
time
at
which
a
second
stimulus
can
produce
a
difference
in
the
mechanical
response
from
that
seen
following
a
single
stimulus
the
dura-
tion
of
the
plateau
may
be
estimated.
The
essential
difference
between
their
experimental
technique
and
ours
is
that
we
have
used
indirect
stimulation.
Initial
assumptions
have
therefore
to
be
made
that
the
time
interval
between
the
application
of
the
second
stimulus
to
the
nerve
and
the
initiation
of
the
muscle
action
potential
remains
the
same
as
that
following
the
first
stimulus,
and
that
the
conduction
velocity
of
the
muscle
action
potential
remains
constant.
With
these
assumptions
it
may
be
seen
from
Fig.
8
that
the
plateau
of
active
state
cannot
last
longer
than
approxi-
mately
3*3
msec
in
the
sarcomeres
of
soleus
muscle
and
2-75
msec
in
the
sarcomeres
of
F.H.L.
In
fact
recent
experiments
(Ridge,
unpublished
observations)
suggest
that,
contrary
to
the
assumption
made
above,
there
is
some
delay
in
the
passage
of
the
second
nerve
action
potential at
very
short
stimulus
intervals,
so
that
the
figures
of
3-3
and
2-75
msec
quoted
above
probably
represent
maximum
estimates.
In
addition,
Dr
Miledi
has
informed
us
that
he
has
observed
curves
comparable
in
shape
to
our
Fig.
8
when
studying
the
responses
of
both
single
nerve
fibres
and
single
muscle
fibres
to
two
stimuli.
He
attributes
the
shape
to
the
interaction
of
the
strength/latency
relation
and
the
relative
refractory
period
of
the
tissue.
If
such
an
interpretation
is
true
in
our
experiments
it
appears
that
we
cannot
define
a
plateau
by
this
method,
either
because
one
does
not
exist
following
a
single
stimulus
(cf.
Walker,
1951)
or
because
its
duration
is
shorter
than
the
limits
set
by
other
factors.
That
the
plateau
of
active
state
in
individual
sarcomeres
of
mammalian
muscle
at
370
C
might
be
as
short
as
2
msec
is
suggested
by
consideration
of
the
Qlo
for
active
state
obtained
in
frog
muscle
by
Sandow
&
Mauriello
(1953)
and
the
relation
between
duration
of
active
state
and
duration
of
absolute
refractory
period
in
frog
muscle
described
by
Ramsey
(1960).
r
Our
estimate
of
the
duration
of
the
plateau
of
active
state
in
fast
skeletal
muscle
is
considerably
shorter
than
that
made
by
Norris
(1960)
using
directly
stimulated
rat
peroneus
digiti
quinti
muscle
at
370
C.
The
differ-
ence
could
be
due
to
the
greater
sensitivity
obtained
by
our
use
of
dif-
ferentiated
tension
records.
The
present
results
cast
some
doubt
on
the
experimental
evidence
brought
forward
by
Bowman,
Goldberg
&
Raper
(1962)
in
support
of
their
contention
that
repetitive
stimulation
of
mammalian
fast
muscle
alters
the
force/velocity
relation
of
the
contractile
element.
These
authors
pointed
out
that
in
their
experiments
the
rate
of
rise
of
tension
in
a
post-
tetanic
twitch
was
greater
than
in
a
preceding
maximal
conditioning
tetanus.
They
argued
that
since
during
a
maximal
tetanus
there
was
(supposedly)
a
constantly
maintained
plateau
of
active
state
the
greater

A.
J.
BULLER
AND
D.
M.
LEWIS
rate
of
tension
development
observed
in
the
post-tetanic
twitch
could
not
be
attributed
to
a
prolongation
of
its
plateau
of
active
state,
as
had
earlier
been
suggested
for
frog
muscle
by
Ritchie
&
Wilkie
(1955).
However,
Bowman
et
al.
(1962)
used
a
stimulation
frequency
of
only
120
pulses/sec
to
produce
their
'maximal'
tetanus.
This
frequency,
as
illustrated
in
Fig.
4,
certainly
does
not
produce
a
fused
contraction
of
fast
muscle
and
would
not
be
expected
to
give
a
maintained
plateau
of
active
state.
Using
higher
tetanic
stimulation
frequencies
(500-600
pulses/sec)
we
have
never
ob-
served
a
post-tetanic
twitch
to
have
a
higher
rate
of
tension
development
than
the
preceding
conditioning
tetanus.
While
our
observations
ob-
viously
still
allow
an
effect
of
repetitive
stimulation
on
the
force-velocity
relation
the
results
of
Bowman,
Goldberg
and
Raper
do
appear
open
to
alternative
interpretation.
Finally,
a
comparison
may
be
made
between
our
differentiated
tension
records
and
those
of
Sandow
(1961).
This
author
has
used
massive
stimula-
tion
of
the
frog
sartorius
muscle
and
thus
obtained
simultaneous
activa-
tion
of
all
the
sarcomeres.
HIis
records
show
a
small
initial
negativity
attributable
to
latency
relaxation,
and
a
hump
on
the
rising
phase
which
is
attributed
to
the
end
of
the
plateau
of
active
state.
With
the
indirect
stimulation
which
we
have
used
the
finite
conduction
velocity
along
the
muscle
fibres
serves
to
blur
out
the
latency
relaxation
of
individual
sarcomeres
and
we
have
seen
no
initial
negativity
in
our
differentiated
tension
records.
The
relatively
slow
conduction
velocity
along
the
muscle
fibres
also
means
that
following
a
single
stimulus
there
will
be
no
'muscle
plateau
of
active
state',
even
if
a
plateau
of
the
value
estimated
above
is
occurring
in
the
individual
sarcomeres.
This
is
because
the
terminal
sarcomeres
of
a
muscle
fibre
will
only
reach
their
plateau
of
active
state
at
a
time
when
the
level
of
active
state
in
the
initially
activated
sarcomeres
is
decaying
owing
to
the
conduction
time
along
the
fibre
exceeding
the
plateau
time.
However,
in
spite
of
the
expectation
that
we
should
not
see
a
hump
on
the
rising
phase
of
our
differentiated
records
we
have
frequently
seen
one,
particularly
when
studying
fast
muscles,
occur-
ring
at
a
time
comparable
to
the
hump
described
by
Sandow.
While
we
have
not
investigated
this
finding
in
detail
we
are
inclined
to
think
that
it
may
be
due
to
minor
inequalities
of
initial
tension
between
different
parts
of
the
muscle.
This
would
equate
the
finding
to
the
much
greater
effects
produced
by
initial
tension
discrepancies
within
the
gracilis
muscle
described
by
Eccles
&
Iggo
(1961).
352

ISOMETRIC
TETANI
OF
MAMMALIAN
MUSCLES
353
SUMMABRY
1.
The
responses
of
soleus
(slow)
and
flexor
hallucis
longus
(fast)
muscles
of
the
cat
have
been
examined
following
both
repetitive
and
double
stimulation
through
the
motor
nerves.
2.
The
maximum
rate
of
rise
of
tension
during
tetanic
stimulation
increases
with
increase
of
stimulation
frequency
to
approximately
300
pulses/sec
in
soleus
and
600
pulses/sec
in
F.H.L.
3.
Two-stimuli
experiments
show
that
the
earliest
observable
separa-
tion
of
the
mechanical
response
following
two
stimuli
from
that
following
one
stimulus
occurs
at
approximately
3-5
msec
after
the
beginning
of
con-
traction
in
soleus
and
approximately
2
msec
in
F.H.L.
4.
These
results
suggest
that
if
a
plateau
of
active
state
is
reached
in
the
sarcomeres
following
a
single
stimulus
its
duration
is
shorter
than
3-5
msec
in
soleus
and
2
msec
in
F.H.L.
We
are
indebted
to
Miss
M.
O'Vens
for
constant
assistance
and
to
Mr
G.
F.
Stonard
for
expert
photographic
help.
We
would
also
like
to
thank
the
Medical
Research
Council
for
a
grant
in
support
of
this
work.
REFERENCES
BOWMAN,
W.
C.,
GOLDBERG,
A. A.
J.
&
RAPER,
C.
(1962).
A
comparison
between
the
effects
of
a
tetanus
and
the
effects
of
sympathomimetic
amines
on
fast
and
slow
contracting
mammalian
muscles.
Brit.
J.
Pharmacol.
19,
464-484.
BROWN,
G.
L.
&
VON
EULER,
U.
S.
(1938).
The
after
effects
of
a
tetanus
on
skeletal
muscle.
J.
Physiol.
93,
39-60.
BROWN,
M.
C.
&
MATTHEWS,
P.
B.
C.
(1960).
The
effect
on
a
muscle
twitch
of
the
back
response
of
its
motor
nerve
fibres.
J.
Physiol.
150,
332-346.
BULLER,
A.
J.,
ECCLES,
J.
C.
&
EccLEs,
R.
M.
(1960a).
Differentiation
of
fast
and
slow
muscles
in
the
cat
hind
limb.
J.
Physiol.
150,
399-416.
BULLER,
A.
J.,
ECCLES,
J.
C.
&
EccLEs,
R.
M.
(1960b).
Interactions
between
motoneurones
and
muscles
in
respect
of
the
characteristic
speeds
of
their
responses.
J.
Physiol.
150,
417-439.
BULLER,
A.
J.
&
LEWIS,
D.
M.
(1963a).
The
rate
of
rise
of
tension
in
isometric
contractions
of
mammalian
fast
and
slow
muscles.
J.
Phy8iol.
169,
29P.
BULLER,
A.
J.
&
LEWIS,
D.
M.
(1963b).
Factors
affecting
the
differentiation
of
mammalian
fast
and
slow
muscle
fibres.
In
Proceedings
of
symposium
on
The
Effect
of
Useand
Disuse
on
Neuromuscular
Functions,
ed.
GUTMANN,
E.
&
HNIK,
P.
Prague:
Czechoslovak
Academy
of
Science.
BULLER,
A.
J.
&
LEWIS,
D.
M.
(1964).
The
rate
of
rise
of
tensioin
in
isometric
tetani
of
cross
innervated
mammalian
skeletal
muscles.
J.
Physiol.
170,
67P.
BULLER,
A.
J.
&
LEWIS,
D.
M.
(1965
a).
Further
observations
on
the
differentiation
of
skeletal
muscles
in
the
kitten
hind
limb.
J.
Phy8iol.
176,
355-370.
BULLER,
A.
J.
&
LEWIS,
D.
M.
(1965
b).
Further
observations
on
mammalion
cross
innervated
skeletal
muscle.
J.
Physiol.
(In
the
Press.)
CHIN,
N.
K.,
COPE,
M.
&
PANG,
M.
(1962).
Number
and
distribution
of
spindle
capsules
in
seven
hindlimb
muscles
of
the
cat.
In
Symposium
on
Muscle
Receptors,
ed.
BARKER,
D.
Hong
Kong:
University
Press.
COOPER,
S.
&
ECCLES,
J.
C.
(1930).
The
isometric
responses
of
mammalian
muscles.
J.
Physiol.
69,
377-385.
ECCLES,
R.
M.
&
IGGO,
A.
(1961).
The
double
twitch
of
gracilis
muscle.
J.
Physiol.
159,
500-506.

354
A.
J.
BULLER
AND
D.
M.
LEWIS
HESS,
A.
&
PILAR,
G.
(1963).
Slow
fibres
in
the
extraocular
muscles
of
the
cat.
J.
Physiol.
169,
780-798.
HILL.
A.
V.
(1949).
The
abrupt
transition
from
rest
to
activity
in
muscle.
Proc.
roy.
Soc.
B,
136,
220-228.
KORN,
G.
A.
&
KORN-,
T.
M.
(1956).
Electronic
Analog
Computers.
New
York:
McGraw-Hill.
KRNJEVIY6,
K.
&
MITCHELL,
J.
F.
(1961).
A
simple
and
reliable
device
utilizing
transistors
for
the
maintenance
of
a
constant
body
temperature.
J.
Physiol.
158,
6P.
MACPHERSON,
L.
&
WILKIE,
D.
R.
(1954).
The
duration
of
the
active
statein
a
muscle
twitch.
J.
Physiol.
124,
292-299.
MOSTOFSKY,
D.
&
SANDow,
A.
(1951).
High
current
dual
pulse
Physiologic
Stimulator.
Electronics
(Nov).
NORRIs,
F.
H.
(1960).
Active
state
plateau
and
latency
in
mammalian
striated
muscle.
Amer.
J.
Physiol. 200,
667-671.
RAMSEY,
R.
W.
(1960).
Some
aspects
of
the
biophysics
of
muscle.
In
Structure
and
Function
of
Muscle,
vol.
II,
ed.
BOIJRNE,
G.
H.
London:
Academic
Press.
RITCHIE,
J.
M.
(1954).
The
duration
of
the
plateau
of
full
activity
in
frog
muscle.
J.
Physiol.
124,
605-612.
RITCHIE,
J.
M.
&
WILKIE,
D.
R.
(1955).
The
effect
of
previous
stimulation
on
the
active
state
of
muscle.
J.
Physiol.
130,
488-496.
SANDow,
A.
(1961).
Energetics
of
muscular
contraction.
In
Biophysics
of
Physiological
and
Pharmacological
Actions.
New
York:
American
Association
for
the
Advancement
of
Science.
SANDow,
A.
&
MAI,RIELLO,
G.
E.
(1953).
Fusion
frequency,
the
kinetics
of
active
state
in
muscular
tetanus.
Fed.
Proc.
12,
123.
WALKER,
S.
M.
(1951).
Tension
and
extensibility
changes
in
muscle
suddenly
stretched
during
the
twitch
response.
Amer.
J.
Physiol.
164,
238-247.
