
M
ICHIGAN DEPARTMENT OF HEALTH AND HUMAN SERVICES
Newborn Screening
Guide for Hospitals
March 2022
TABLE OF CONTENTS
Introduction ............................................................................................................................................... 1
Overview of Michigan Newborn Screening .............................................................................................. 2
Dried Blood Spot Screening ................................................................................................................. 2
Hearing Screening ................................................................................................................................ 3
Critical Congenital Heart Disease Screening ....................................................................................... 3
Newborn Screening Practice and Procedure .......................................................................................... 4
Role of the Newborn Screening Coordinator ....................................................................................... 4
Completing the Newborn Screening Card ........................................................................................... 5
Infant Information............................................................................................................................ 5
Mother Information ......................................................................................................................... 7
Provider Information ....................................................................................................................... 7
Submitter Information .................................................................................................................... 8
Expiration Date: ............................................................................................................................... 8
Notes Field: ...................................................................................................................................... 8
Internal Use Labels: ........................................................................................................................ 9
Closed Adoption and Safe Surrender: ......................................................................................... 9
Notifying NBS Follow-up of Changes to Infant’s Health or Guardian Status: ........................ 9
Recording the NBS Card Number ...................................................................................................... 10
Michigan BioTrust for Health .............................................................................................................. 11
BioTrust Consent Form Instructions .......................................................................................... 11
Ordering Newborn Screening Cards, Return Envelopes and Brochures .......................................... 12
Specimen Collection ........................................................................................................................... 13
Laboratory Testing Methods ............................................................................................................... 13
Disorders Identified in Michigan Newborn Residents via Newborn Screening, 1965-2019 ............. 14
Quality Assurance ............................................................................................................................... 15
NICU Protocol (includes Special Care Nursery) ................................................................................ 16
Transfusions .................................................................................................................................. 16
Early Specimens ........................................................................................................................... 17
Total Parenteral Nutrition (TPN) .................................................................................................. 17
Transferred Newborns.................................................................................................................. 17
Newborn at High Risk of Having a NBS Disorder ..................................................................... 18
Newborn Death or Pending Death .............................................................................................. 18
Primary Care Provider Information ........................................................................................................ 19
Follow-up of Positive NBS Results ..................................................................................................... 19
NBS Result Request Policy ................................................................................................................ 19
Questions on Positive Reports Received ........................................................................................... 19
Documentation of NBS Results .......................................................................................................... 20
Missing Bloodspot Newborn Screens ................................................................................................ 20
Frequently Asked Questions .................................................................................................................. 22
Resource List .......................................................................................................................................... 23
Contact Information ................................................................................................................................ 24
NBS Follow-up .................................................................................................................................... 24
Contact Information ................................................................................................................................ 25
NBS Lab .............................................................................................................................................. 25
Contact Information ................................................................................................................................ 26
Early Hearing Detection and Intervention .......................................................................................... 26
Contact Information ................................................................................................................................ 27
NBS Hepatitis B .................................................................................................................................. 27
Contact Information ................................................................................................................................ 28
NBS Follow-up Coordinating Centers ................................................................................................ 28
Contact Information ................................................................................................................................ 29
Courier Services ................................................................................................................................. 29
Lower Peninsula............................................................................................................................ 29
Upper Peninsula ............................................................................................................................ 29
Appendix 1 – Legislative Mandates ....................................................................................................... 30
Public Health Code Act 368 of 1978 .................................................................................................. 30
Act No. 31, Public Acts of 2006 to amend 1978 PA 368 ................................................................... 30
Appendix 2 – Blood Specimen Collection and Handling Procedure ..................................................... 31
Appendix 3 – NBS Card Images ............................................................................................................ 32
Appendix 4 – Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule ................... 33
Appendix 5 – NBS Online Ordering System (NBSO) ............................................................................ 34
Appendix 6 – Newborn Screening Card Replacement Form ................................................................ 35
Appendix 7 – NBS Follow-up Program Hospital Discharge Sheet ....................................................... 36
Appendix 8 – Disorder List ..................................................................................................................... 37
Amino Acid Disorders ..................................................................................................................... 37
Fatty Acid Oxidation Disorders ....................................................................................................... 37
Organic Acid Disorders ................................................................................................................... 37
Hemoglobinopathies ....................................................................................................................... 37
Endocrine Disorders ....................................................................................................................... 37
Lysosomal Storage Disorders......................................................................................................... 37
Other Disorders ............................................................................................................................... 37
Disorder Coming Soon.................................................................................................................... 37
Appendix 9 – Specimen Directives ........................................................................................................ 38
Appendix 10 – NICU and Special Care Nursery Algorithm ................................................................... 39
Appendix 11 – Fax Reporting ................................................................................................................ 40
Appendix 12 – Military Time ................................................................................................................... 42
Appendix 13 – NBS Report Request Form ............................................................................................ 43
Appendix 14 – Weight conversion Chart ............................................................................................... 44
Appendix 15 – Missing Bloodspot Specimen Process .......................................................................... 45
1
INTRODUCTION
Newborn screening (NBS) saves lives and protects the health of Michigan newborns. Since 1965, all
Michigan newborns have been screened shortly after birth to determine if they are at risk for having
rare but treatable genetic disorders. If untreated, these disorders can lead to illness, physical
disability, intellectual disability or death. Medication and changes in diet can help prevent many
health problems caused by disorders detected by NBS.
Whether your role is as a primary care provider, neonatologist, pediatric or neonatal nurse
practitioner, nurse clinician, nurse, laboratory professional, administrator or support staff member,
you play an important role in NBS. Most primary care providers will, at some point, receive notice of
an abnormal newborn screen. Neonatal intensive care unit (NICU) staff members are much more
likely to deal with abnormal NBS results. Nursery staff will be involved in the follow-up of abnormal
results, collection of repeat specimens, and assurance that all infants in their units have documented
NBS results. While the disorders included in the NBS panel are individually rare, approximately 270
Michigan newborns are identified with these disorders each year. This NBS guide is intended to be a
reference tool and contains background information, general guidance on common issues related to
NBS, specific forms, and contact information.
It is important to recognize that NBS, within the hospital setting, requires specific administrative
guidelines to address Michigan Department of Health and Human Services (MDHHS) NBS Program
specimen collection, handling and transit time procedures. The appointment of a hospital NBS
coordinator is necessary for quality assurance and ongoing coordination within your hospital
and with the State’s NBS program. The coordinator may be a nurse manager, unit nurse,
laboratory technician, clerk or secretary who will coordinate quality assurance activities within the
hospital and between the hospital and the NBS Program.
The hospital based NBS program must include an internal hospital NBS protocol. Specific
information each hospital should include in the protocol are instructions/materials for:
1. Maintaining an inventory of NBS supplies such as NBS cards, forms, and educational
materials.
2. Educating hospital nursery and NICU staff on the NBS specimen collection protocol.
3. Recording and entering the NBS card (‘kit’) number on the electronic birth certificate (EBC).
4. Maintaining tracking logs on NBS specimen collection, courier pickup, and screening results.
5. Courier pickup for delivery of NBS specimens to the MDHHS NBS Laboratory.
6. Ongoing education of hospital laboratory and nursery staff regarding the NBS process within
the hospital and any NBS program changes.

2
OVERVIEW OF MICHIGAN NEWBORN SCREENING
Dried Blood Spot Screening
Michigan is a leader in NBS and now screens for more than 50 disorders plus hearing loss and
critical congenital heart disease. Appendix 8
contains a complete list of disorders on the NBS panel.
Michigan law mandates NBS. Appendix 1 summarizes NBS legislation. If parents will not permit
collection of the screen, it is suggested that the hospital request that parents sign a waiver stating
that they were informed of the risk to their newborn is screening is declined and return the form to the
NBS Program.
Before a newborn is discharged from the hospital, a blood specimen is collected on a NBS card
purchased from the NBS Program. A picture of the current NBS card is included in Appendix 3
.
Blood specimens should be collected at 24-36 hours of life, ideally 24-30 hours, and air dried a
minimum of three hours. The NBS Program provides courier service to every hospital Monday
through Friday. Hospitals in the Upper Peninsula have courier service on Saturday and those in the
Lower Peninsula on Sunday. Blood specimens should be sent each day courier service is available.
Laboratory testing is typically completed within one or two days of specimen receipt, and all NBS
results are faxed or mailed to the hospital that submitted the specimen. The NBS Laboratory would
like all hospitals to receive NBS results by fax to assure prompt receipt. Appendix 11
contains
instructions on how to receive faxed NBS results. The NBS Laboratory operates Monday through
Saturday.
The NBS Program is unable to perform stat laboratory testing. If you are caring for a newborn who
has been previously screened and subsequently develops an acute metabolic crisis, it is appropriate
to contact the NBS Program to obtain screening results. However, if a newborn is suspected of
having a disorder that is included in the NBS panel, the newborn should be clinically evaluated rather
than assume that screening results will be available with the rapidity required in an emergency
situation. Sub-specialists from each NBS follow-up coordinating center
are available for guidance in
such circumstances.
The NBS Program will notify the primary care provider (or NICU) identified on the specimen card if
the specimen is:
• Positive for a disorder
• Unsatisfactory for testing
• Early (collected before 24 hours of life)
When a newborn screen is a strong positive for a disorder, the NBS Program will contact the primary
care provider or NICU by fax. In addition, the primary care provider will be contacted by the
appropriate coordinating center to arrange for confirmatory testing, diagnosis, and treatment.

3
Hearing Screening
Approximately 150 newborns with hearing loss are identified annually by newborn hearing screening
in Michigan. NICU infants are at increased risk for hearing loss compared to the general newborn
population. Hearing screening of newborns who are premature, ill, or have birth defects can be
problematic due to confounding factors presented by their conditions and the treatment required.
Michigan has instituted a mandated screening and reporting system for universal newborn hearing
screening. The first goal of the hospital-based program is to screen all newborns no later than one
month of age. Newborns who exhibit evidence of hearing loss should have a hearing assessment by
an audiologist no later than three months of age and early intervention services initiated no later than
six months of age. Hearing screening should be completed no later than one month of age through
either of the following methods: otoacoustic emissions (OAE) or automated auditory brainstem
response (AABR). Each hospital should have a hearing screening protocol in place. When the Early
Hearing Detection and Intervention (EHDI) Program is informed about a newborn who does not pass
the hearing screen, notification is sent to the primary care provider. Please contact EHDI
to receive
information on how to help ensure timely follow-up for newborns.
Critical Congenital Heart Disease Screening
Congenital heart defects are the most common group of birth defects, affecting 9 in 1,000 newborns.
Critical congenital heart diseases (CCHDs) are those requiring surgery or catheter intervention in the
first year of life. CCHDs remain one of the most significant causes of infant death in the United
States.
Effective April 1, 2014, the Michigan Department of Health and Human Services (MDHHS) has
mandated statewide pulse oximetry screening of all Michigan newborns for CCHDs prior to hospital
discharge. The NBS Program and the CCHD Advisory Committee recommend that newborns be
screened as close to 24 hours of age as possible, using the approved MDHHS CCHD Screening
Algorithm.
The NBS Program evaluates each hospital’s critical congenital heart disease (CCHD) screening
performance and provides a quarterly report to each hospital that specifies if the following selected
targets have been met:
1. Greater than 90% of newborns with a bloodspot screen have a right hand and foot pulse oximetry
screen reported to the state.
2. Greater than 90% of newborns with a bloodspot screen have a right hand and foot pulse oximetry
screen reported to the state less than 10 days after screen date.
3. Greater than 90% of newborns with a bloodspot screen have a right hand and foot pulse oximetry
screen completed between 20 and 28 hours after birth.
More information about CCHD screening, data reporting, and educational materials for healthcare
providers and parents is available on the newborn screening CCHD
website (Michigan.gov/cchd). The
CCHD Guide for Hospitals is located under the Provider Resources section.
4
NEWBORN SCREENING PRACTICE AND PROCEDURE
Role of the Newborn Screening Coordinator
The hospital NBS coordinator plays a crucial role in assuring that the NBS process is both effective
and efficient. The coordinator fulfills this role by:
1. Knowing how the newborn nursery/NICU, hospital laboratory, and mailroom interact in the
NBS process.
2. Assisting NBS program staff in resolving problems.
The suggested responsibilities of the NBS coordinator are:
1. Perform quality assurance activities:
a. Assure that there is a NBS protocol in place describing the hospital’s NBS policies and
procedures.
b. Assure that a log is maintained to track NBS specimens, courier pickup, and receipt of
screening results.
c. Assure adequate inventory of NBS cards.
d. Ensure expired cards are removed from stock.
e. Provide guidance/information to nursery and laboratory staff on the importance of
accurately filling out all demographic fields on the NBS card.
f. Assist NBS program staff in resolving problems of missing/incorrect demographic
information on the NBS card and in obtaining repeat samples when specimens are
unsatisfactory for testing.
g. Assure that hospital NBS policies and procedures include a protocol for notifying the
NBS program if a newborn death occurs after a specimen was sent to the NBS
laboratory.
2. Perform education activities:
a. Serve as a contact person and facilitator between the NBS Program and hospital staff
involved in the NBS process to:
i. Inform and educate hospital staff about new program guidelines and protocol
changes (new disorders added to test panel, changes in specimen collection
requirements, and other NBS information, as necessary).
ii. Disseminate information (newsletters, quality assurance (QA) report) received from
the MDHHS NBS Program to appropriate hospital staff (nursing, laboratory,
clinicians).
b. Assure that there is an adequate supply of NBS and BioTrust brochures and a
mechanism for distribution to all mothers.
c. Work with obstetrical department staff to incorporate NBS and BioTrust educational
information in existing and future prenatal classes offered to parents.
d. Attend trainings offered by the NBS Program.

5
Completing the Newborn Screening Card
It is extremely important to fill out the NBS card completely and accurately. Inaccurate information
can lead to critical delays in identifying and reporting of abnormal results. Press firmly using a black
or blue pen and clearly print the information. The card will be scanned into the NBS database, so
legibility is critical. The specimen submitter is legally responsible for the accuracy and completeness
of the information on the NBS card. The Completing the Newborn Screening Card infographic is a
simple tool to help hospital staff to correctly complete the demographic section of the card. This
resource is available at the newborn screening
website (Michigan.gov/newbornscreening) in the
“Resources for Hospitals and Health Professionals” section. Include the following information in the
spaces provided on the NBS card:
Infant Information
INFANT’S NAME: Record last name followed by first name. If no first name is available at
the time of specimen collection, the last name followed by “boy” or “girl” should be used. For
single mothers, use the last name of mother or last name specified by mother. DO NOT
LEAVE BLANK.
SEX: Completely shade in the appropriate oval to designate newborn’s sex as male, female,
or ambiguous.
BIRTH DATE: Use a six-digit number (mm/dd/yy) for date of birth. For example, a birth on
January 4, 2021 would be recorded as 011421.
BIRTH TIME: Record time of birth in military time. For example, a birth at 4:30 p.m. would be
recorded as 1630. For help with time conversions, see Appendix 12.
BIRTH WEIGHT (grams): Record the birthweight in grams in the boxes provided. DO NOT
use pounds and ounces. Accuracy is critical as lab cutoff levels can be dependent on weight.
Note: Birth weight is required on the first sample (“blue”) card only. For help with weight
conversions, see Appendix 14.
CURRENT WEIGHT (grams): Record the current weight in grams in the boxes provided. Do
not use pounds and ounces. Note: Current weight is required on the repeat sample (“pink”)
card only. For help with weight conversions, see Appendix 14.
WEEKS GESTATION: Record weeks of gestation at time of birth. Note: This information is
requested for the first sample (“blue”) card only. It is not necessary to add this information to
the repeat sample (“pink”) card.
SINGLE BIRTH: Completely shade in oval for single birth.
MULTIPLE BIRTH ORDER: Completely shade in oval to record birth order by “A”, “B”, “C” for
twins, triplets, etc.
ANTIBIOTICS: Mark ‘yes’ next to antibiotics if the newborn received postnatal antibiotics
prior to the first sample specimen collection or is currently receiving antibiotics at the time of a
repeat sample collection. Do not check antibiotics if the newborn received antibiotics in the
past but has not received them within 48 hours of collection. It is no longer necessary to
include information about the mother’s perinatal antibiotic use.

6
COLLECTION DATE: Use a six-digit number (mm/dd/yy) representing the date on which the
specimen was collected. Previously referred to as specimen date.
COLLECTION TIME: Record time of specimen collection in military time. For help with time
conversions, see Appendix 12
.
COLLECTED BY: Record initials or employee hospital identification number of the person
collecting the specimen.
NICU/SPECIAL CARE: Indicate if the newborn was in the NICU or special care nursery at
the time the specimen was collected. If neither, completely shade in the oval next to “no”.
RBC TRANSFUSION: Completely shade in oval “no” or “yes” to indicate whether the
newborn was ever transfused with red blood cells prior to specimen collection, including in
utero. If yes, give date (mm/dd/yy) and the start time (military) of the most recent transfusion.
For example, if the transfusion started on October 13, 2021 at 11:20 p.m., enter 101321 2320.
MEDICAL RECORD NUMBER BABY: Record the birth hospital’s identification or medical
record number. Note that laboratory data coders are unable to enter letters, hyphens and
spaces that appear in a medical record number.
ANY TPN FEEDING: Completely shade in oval “yes” if the newborn is receiving total
parenteral nutrition (TPN) at the time the specimen is collected – OR – received TPN within
24 hours of specimen collection.
ETHNICITY: Completely shade in oval for Hispanic or non-Hispanic. Ethnicity should be filled
in first and, in addition, one of the six boxes for race should be filled in. Mark the mother’s
ethnicity if the father’s ethnicity is unknown. Note: Ethnicity information is requested for the
first sample (“blue”) card only.
RACE: Completely shade in the oval for one of the six racial categories after the designation
of Hispanic or non-Hispanic has been selected. If the newborn has a parent in one racial
category and the other parent is in a different racial category, fill in the multi-Racial oval. It is
very important to fill in either the Hispanic or non-Hispanic box and in addition fill in one of the
six boxes for race. Mark the mother’s race if the father’s race is unknown.
Example 1: One parent identifies as Hispanic and both parents identify as Black. The card should be
marked Hispanic and Black.
Example 2: One parent identifies as Hispanic and White; the other parent identifies as non-Hispanic
and Black. The card should be marked Hispanic and Multi-Racial.
Example 3: Neither parent identifies as Hispanic. One parent identifies as White; the other parent
identifies as Asian. The card should be marked non-Hispanic and Multi-Racial.
TYPE OF COLLECTION: The preferred collection method is by heel stick with a single drop
of blood applied directly to each circle on the filter paper. Note that the use of capillary tubes
can result in layered, serum, clotted, or damaged specimens. If the heel was not used,
indicate the alternate collection method. The type of flush refers to the flush used prior to
specimen collection, such as heparin, saline, or none.

7
OTHER FEEDING: Check all that apply. For instance, if a mother is both breast and bottle
feeding, mark both and indicate the type of formula. It is no longer necessary to include
information about use of human milk fortifier.
Mother Information
MOTHER’S NAME: Record last name followed by first name as it will appear on the
newborn’s birth certificate. If the newborn is not going to be released to the care of the mother
at birth, mark adoptive parent, foster parent, or adoption agency next to ‘If Not Birth Parent’.
Please provide the contact information for the adoptive parent, foster parent, or adoption
agency in place of the mother’s information* (refer to the HBsAg section on how to document
the birth mother’s HBsAg test date and result). Do not place sticky notes on the card or use
red ink. Neither will be recorded when the card is scanned into the laboratory information
management system. If contact information on new parents, foster parents, or the adoption
agency is not on the card, we will not be able to contact the family if necessary. We would like
to avoid calling the birth mother if she is no longer responsible for the care of the newborn.
MOTHER’S ADDRESS: Record mother’s current street address, apartment/unit/lot number,
followed by city, state and zip code. Information about the mother is needed to locate
newborns in need of clinical evaluation or retesting.
MOTHER’S PHONE: Record mother’s area code and primary telephone number.
MEDICAL RECORD NUMBER – MOTHER: Record the hospital identification or medical
record number. Note: This information is only required on the “blue” first sample card.
Laboratory data coders are unable to enter letters, hyphens and spaces that appear in a
medical record number.
BIRTH DATE: Record the mother’s date of birth (mm/dd/yy).
HEPATITIS B SURFACE ANTIGEN (HBsAg): Provide date of test (mm/dd/yy) and
completely shade in the appropriate oval to indicate a positive or negative result. If there is no
HBsAg test result in the mother’s record, the test should be done immediately. Positive
HBsAg results should be faxed to the MDHHS Perinatal Hepatitis B Prevention Program at
517-763-0470. This important information helps assure that infants at risk receive the proper
interventions. Note: HBsAg information is requested for first sample (“blue”) cards only. *If
“Not Birth Parent”, the birth mother’s HBsAg results would not be documented under this
section. However, if the birth mother’s HBsAg result is positive, please contact the PHBPP at
517-242-8319.
Provider Information
PROVIDER’S NAME: Record last name, followed by first name, of the primary care provider
(PCP) to be notified of an unsatisfactory or positive newborn screen. At the time of collection,
verify with the mother that the PCP’s name entered on the card is correct. If the mother does
not offer a PCP’s name, the physician in charge of the newborn nursery should be listed on
the NBS card. The physician should arrange for all retesting through the hospital’s outpatient
laboratory. If the newborn is expected to be in the NICU for at least a week, list a staff
neonatologist as the physician and write the NICU telephone and fax numbers on the NBS
card. If discharge is expected within a week, write the name and clinic telephone and fax

8
numbers of the provider who will be taking care of the newborn after discharge. DO NOT
LEAVE BLANK.
PROVIDER’S PHONE: Indicate the primary care provider’s area code followed by the
telephone number. It is very important to provide a complete and correct number. This
information is used to contact the primary care provider with positive screen results and
follow-up information. If the hospital newborn nursery chooses to follow-up positive results
directly, provide the name and telephone number of the staff person designated to contact the
family. This option is preferred for newborns without a designated primary care provider.
PROVIDER’S FAX: Indicate the primary care provider’s area code followed by fax number.
The fax number is needed to forward to the provider screening results that require further
follow-up.
Submitter Information
SUBMITTER NAME: Record the name of the submitter (this should be the birth hospital or
midwife on all first sample newborn screens). If abbreviation of the hospital’s name is
necessary, use some letters from each word in the hospital’s name. For example, the
abbreviation for St. Joseph Mercy Hospital would be St. Jos. Mrcy. It is acceptable to apply a
pre-printed hospital label that includes the hospital name, address, telephone number, and
the appropriate hospital code.
HOSPITAL CODE: MDHHS has assigned a 3-digit hospital code for each hospital that must
be recorded in the boxes provided. The 3-digit code should be listed before the two preprinted
zeros. For regular nurseries, a “0” should be added to the last box (after the two preprinted
zeros). For the NICU, a “1” should be added to the last box. For the special care nursery, a
“2” should be added to the last box.
SUBMITTER ADDRESS: Record the submitter’s street address followed by the city, state
and zip code.
SUBMITTER PHONE: Record submitter’s area code and telephone number.
BIRTH HOSPITAL: Record name of the birth hospital here only if different from the
submitter.
Expiration Date:
EXPIRATION DATE: The expiration date is located on the middle of the right-hand side on
the newest cards and in the lower right-hand corner of older cards. Check the expiration date
each time you collect a blood spot specimen. Cards used after the expiration date will be
marked ‘unsatisfactory/expired card’ and a repeat specimen will be requested.
Notes Field:
NOTES FIELD: The Notes field added to the lower right-hand corner of newer cards can be used to
notify the NBS Program of information such as newborn transfer, family history of a disorder,
meconium ileus, the mother’s name in the event the baby will not be released to her care, etc.

9
Internal Use Labels:
INTERNAL USE LABELS: If internal hospital/lab use labels are placed on the NBS
demographics card, do not affix to any area designated as DHHS Use Only. Labels can be
placed on the back of the card or in the notes section.
Closed Adoption and Safe Surrender:
INFANT’S NAME: Record as “Baby Doe” or as the hospital generated name used in hospital
medical record.
INFANT’S BIRTH DATE/TIME: If the date of birth is unknown, use the surrender date and
time.
MOTHER’S SECTION:
o Mark the appropriate circle for the information provided. This may be adoptive parent,
foster parent, or adoption agency.
o Name and contact information should reflect who will be caring for the infant upon
release. If the adoptive or foster parent information is not available, please place the
name and contact information for the adoption agency.
o Mother’s medical record, birth date, and Hepatitis B Surface Antigen results can be left
blank for these cases.
NOTES SECTION: Adding a note in the lower right-hand corner of the card indicating this is
a closed adoption or safe surrender will help ensure that this is clearly recorded in our system.
ADOPTION DECISION MADE AFTER NBS CARD SUBMITTED: If the NBS card has
already been sent to MDHHS with birth mother’s information and then the decision is made to
place the infant into closed adoption or safe surrender, please notify us immediately and
request that the birth mother’s information is removed from our system.
o Notification can be faxed utilizing the NBS Follow-up Program Hospital Discharge
Sheet found in Appendix 7
or by calling the NBS Follow-up Program at 517-335-4181.
Notifying NBS Follow-up of Changes to Infant’s Health or Guardian Status:
It is very important that you notify NBS Follow-up of any changes to the infant’s status that
occurred after you sent the infant’s blood spot specimen to the laboratory. Complete and
return the NBS Follow-up Program Hospital Discharge Sheet found in Appendix 7
.
Note: It is extremely important to fill out the screening card completely and accurately.

10
Recording the NBS Card Number
The hospital NBS protocol should include instructions to ensure that the NBS card number is
forwarded to the staff person responsible for submitting the electronic birth certificate (EBC). The
NBS card (“kit”) number is referred to as the “metabolic number” on the EBC. This number is in the
lower right-hand side of the card above the barcode (as shown below) and goes in the upper right-
hand box on the EBC.

11
Michigan BioTrust for Health
The Michigan BioTrust for Health (BioTrust) is a program that oversees the storage of residual dried
blood spots (DBS) from NBS for their potential use in medical and public health research. Hospital
staff should provide the BioTrust consent brochure entitled After Newborn Screening, Your Baby’s
Blood Spots to parents and ask if they are willing to grant permission to make their infant’s DBS
available for health research once NBS is complete. Permission is granted by marking the “yes”
check box and signing the consent form located on the back of the NBS first sample card. If parents
decline permission for the BioTrust, please have them mark the “no” checkbox and sign the BioTrust
form. MDHHS staff, upon request, will provide onsite training on the BioTrust and the parental
consent process. This training is also available on the newborn screening
website
(Michigan.gov/newbornscreening) within the Resources for Hospitals and Health Professionals
section.
Appendix 5 contains information on how to obtain After Newborn Screening, Your Baby’s
Blood Spots consent brochures.
If a parent declines the BioTrust, his/her newborn’s DBS will still be stored for up to 100 years unless
the parent requests that the specimen be destroyed. Parents who would like to have their newborn’s
DBS destroyed should sign and return the Residual Newborn Screening Blood Spot Directive form. If
a parent is comfortable with his/her newborn’s DBS being stored but not made available for research,
no additional steps are necessary other than marking the “no” checkbox and signing the BioTrust
consent form located on the back of the NBS first sample card.
Residual DBS of persons born after July 1984 and prior to May 2010 are currently stored and
available for research through the BioTrust. Persons over the age of 18 or parents of minor children
who would like to have these samples destroyed must sign and return the Residual Newborn
Screening Blood Spot Directive form. Persons over the age of 18 or parents of minor children who
would like these samples to remain in storage but no longer made available for research must sign
and return the Residual Newborn Screening Blood Spot Directive form. Appendix 9
contains this
form.
BioTrust Consent Form Instructions
1. Provide the Michigan Newborn Screening Saves Babies brochure and the After Newborn
Screening, Your Baby’s Blood Spots BioTrust consent brochure to parents. Clarify the
difference between the mandatory NBS Program and the optional Michigan BioTrust for
Health, which allows residual DBS to be used for research.
2. Inform parents about the Michigan Newborn Screening Saves Lives video and that it can be
viewed either on the newborn screening
website (Michigan.gov/newbornscreening) within the
General Information for Families section or through your hospital TV channel, if available.
3. Complete the demographic information on the front of the NBS first sample card and collect
the blood specimen as usual. The BioTrust consent form for residual DBS use is attached to
the back of the NBS first sample card
. If parents are undecided or not available to make a
decision about granting consent for the BioTrust at the time the NBS specimen is collected,
remove the consent form for later use. Hospital staff should write the baby’s name or affix the
patient label on the back of the white copy of the consent form to keep track of the form more
easily after it has been separated from the card.
Note: Each NBS card has the same unique ID number on all pages, including the BioTrust
consent form. This number is used to link a baby’s NBS specimen to the parent’s BioTrust
consent form if received at a later time in the NBS Laboratory.

12
4. Prior to obtaining consent, confirm that parents have received the NBS brochure and BioTrust
consent brochure:
• The Michigan Newborn Screening Saves Babies brochure explains NBS and
introduces the Michigan BioTrust for Health.
• The After Newborn Screening, Your Baby’s Blood Spots BioTrust consent brochure
details possible research use of residual DBS and information needed for parents to
decide whether to grant permission for use of these DBS for research.
5. If parents wish to allow use of their newborn’s residual DBS for research, ask one parent to
mark the “yes” checkbox and sign the white copy of the BioTrust consent form located on the
back of the first sample (“blue”) card.
• If consent is not granted, ask one parent to mark the “no” checkbox and sign the white
copy of the BioTrust consent form. Return the white copy to the NBS Laboratory once
the parent marks his/her decision and signs the consent form.
• The bottom pink copy is for the parent to keep.
6. Submit the white copies of the BioTrust consent form in the same envelopes used for DBS
specimen cards.
Note: A consent form does not need to be in the same envelope as the newborn’s NBS
specimen card. DO NOT delay returning a newborn’s NBS specimen card while waiting for
the consent form.
7. If parents wish to allow use of their newborn’s residual DBS for research, ask one parent to
mark the “yes” checkbox and sign the white copy of the BioTrust consent form located on the
back of the first sample (“blue”) card.
• If consent is not granted, ask one parent to mark the “no” checkbox and sign the white
copy of the BioTrust consent form. Return the white copy to the NBS Laboratory once
the parent marks his/her decision and signs the consent form.
• The bottom pink copy is for the parent to keep.
• Only the parent or legal guardian can sign the consent form. In cases of adoption, the
birth mother could sign the consent form while she remains the legal guardian.
However, the future adoptive parent may wish to select a different option. An option is
to return the NBS specimen without the BioTrust consent form. Then send the form
and brochure home with the family and/or adoption agency caring for the infant. Once
legal guardianship is established, that person can make the BioTrust decision and
return the form to MDHHS.
Ordering Newborn Screening Cards, Return Envelopes and Brochures
Hospital and health system supply purchasing personnel should use the NBS Online Ordering
System (NBSO) to order NBS cards.
Replacement cards are available free of charge for any card that cannot be used. Reasons for
replacement could include card pieces are torn or separated, the specimen is unsatisfactory for
testing, the wrong demographic information was entered on the card, etc. Complete the
card
replacement form and return that and the white face sheet(s) of the cards intended for replacement
to the address on the form. DO NOT send these requests to the State NBS Laboratory. Failure to
send the request to the address on the form will result in delay and could result in no replacement
cards being issued.

13
NBS brochures are free of charge and shipped in quantities of 50 (English version) and 25 (Spanish
and Arabic versions). They can be ordered through NBSO free of charge. For ordering, please visit
the newborn screening ordering website (Michigan.gov/nbso).
Specimen Collection
• Direct specimen collection from a heel puncture is preferred for optimal laboratory results.
Blood collection using capillary tubes is discouraged.
• First sample specimens should be collected between 24-36 hours of age, preferably 24-30
hours.
• Specimens should be air dried in a horizontal position for a minimum of three hours and sent
by courier
o the same day if collected more than five hours before the scheduled courier pickup
time that day.
o or the next day the courier is scheduled to pick up specimens if collected less than five
hours before the scheduled courier pickup time that day.
• Tips for avoiding unsatisfactory specimen collection can be found at the newborn screening
website (Michigan.gov/newbornscreening) in the “Resources for Hospitals and Health
Professionals” section.
Laboratory Testing Methods
• Tandem Mass Spectrometry (MS/MS): Amino acid, organic acid and fatty acid oxidation
disorders are detected by evaluation of specific MS/MS acylcarnitine and amino acid profiles.
If a screen is positive for propionic acidemia/methylmalonic acidemia, homocystinuria or
malonic acidemia, a secondary screen is performed by the Mayo Biochemical Genetics
Laboratory.
• Fluoroimmunoassay (FIA): Congenital hypothyroidism (CH), congenital adrenal hyperplasia
(CAH), and cystic fibrosis (CF) are detected by FIA for thyroid stimulating hormone (TSH), 17-
hydroxyprogesterone (17-OHP) and immunoreactive trypsinogen (IRT), respectively. If a
screen is positive for CAH, a secondary screen for CAH by steroid profile is performed by
MS/MS by the Mayo Biochemical Genetics Laboratory. A secondary DNA screen for 60 CF
mutations is performed by the NBS Laboratory on specimens with IRT values ≥96
th
percentile.
• High Performance Liquid Chromatography (HPLC) and Isoelectric Focusing (IEF):
Hemoglobinopathies, including sickle cell anemia, sickle/beta thalassemia, hemoglobin SC
disease and hemoglobin H disease, are detected by HPLC (primary screen) and further
differentiated by IEF (secondary screen).
• Enzyme assays: Galactosemia (galactose-1-phosphate uridyltransferase) and biotinidase
deficiency (biotinidase).
• Digital Microfluidics: Pompe disease and Mucopolysaccharidosis Type I (MPSI) are
screened using a digital microfluidics platform. If a screen is positive for Pompe disease or
MPSI, a secondary screen is performed by the Mayo Biochemical Genetics Laboratory.

14
Disorders Identified in Michigan Newborn Residents via Newborn
Screening, 1965-2019
Type of Disorder Classification
(Year Screening Began)
Cases in
2019
(N)
Cases
Through 2019
(N)
Cumulative
Detection
Rate
Galactosemia (1985)
9
219
1:20,436
Biotinidase deficiencies (1987)
8
360
1:11,667
Amino acid disorders (1965)
13
793
1:9,375
Organic acid disorders (2005)
9
100
1:17,205
Fatty acid oxidation disorders (2003)
12
287
1:6,903
Congenital hypothyroidism (1977)
126
2585
1:1,625
Congenital adrenal hyperplasia (1993)
5
175
1:19,002
Sickle cell disease (1987)
64
2092
1:2,004
Hemoglobin H disease (2012)
4
16
1:55,110
Cystic fibrosis (October 2007)
16
322
1:4,165
Primary immunodeficiencies (October 2011)
12
120
1:8,214
Lysosomal storage disorders (August 2017)
5
17
1:15,297
X-linked adrenoleukodystrophy (October 2019)
1
1
1:25,710
Total
284
7,092
-
15
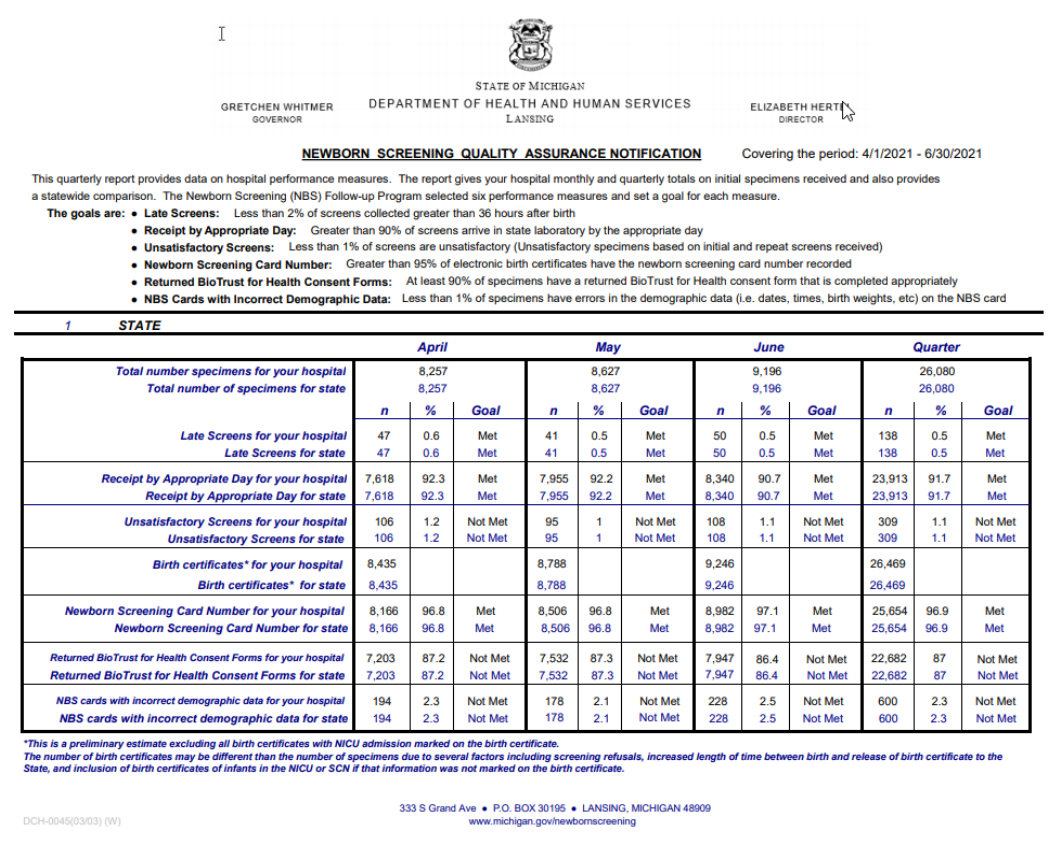
Quality Assurance
The NBS Program evaluates each hospital’s NBS performance and provides a quarterly report to
each hospital that specifies if the following selected targets have been met:
1. Less than 2% of screens collected greater than 36 hours after birth.
2. Greater than 90% of screens arrive in State laboratory by the appropriate day.
3. Less than 1% of first and repeat sample specimens unsatisfactory for testing.
4. Greater than 95% of electronic birth certificates have the newborn screening card number
recorded.
5. At least 90% of specimens have a returned BioTrust for Health consent form that is completed
appropriately.
6. Less than 1% of specimens have errors in the birthdate/time, specimen collection date/time,
birthweight in grams, and/or transfusion status on the NBS card.
An example of a quarterly Newborn Screening Quality Assurance Notification appears below:

16
NICU Protocol (includes Special Care Nursery)
First sample NBS specimens for all NICU newborns, regardless of birth weight, should be collected
at 24-30 hours of life and prior to red blood cell (RBC) transfusion. The NICU protocol
contains
guidelines to follow under certain circumstances. A repeat sample specimen is required at 30 days,
or at discharge for any newborn hospitalized 8-30 days. This includes babies who have transferred
from the NICU to a general floor without having been discharged home. If a newborn was discharged
home before 8 days, had a normal first sample screen, and is later readmitted, a repeat sample
specimen does not need to be collected. The NBS Program considers the day the baby is born as
day of life one.
It is not necessary to repeat the NICU protocol if a baby is discharged from the NICU and is later
readmitted.
It is not necessary to wait until discharge to obtain a repeat sample specimen. If a NBS disorder is
suspected, a NBS specimen can be ordered by the physician, collected, and forwarded to the NBS
Laboratory at any time prior to discharge. Follow the instructions on the notification letter for
obtaining an additional specimen if you are informed that a previous specimen was positive, early, or
unsatisfactory.
Transfusions
For the typical NICU newborn, the first sample specimen should be collected between 24-30 hours of
life and prior to RBC transfusion. A repeat sample specimen should be collected at discharge or 30
days of life, whichever comes first.
In those rare cases when the first sample specimen cannot be collected prior to RBC transfusion
• a first sample specimen should be collected 28 hours post RBC transfusion start time.
• a repeat sample specimen should be collected at discharge or 30 days of life, whichever
comes first.
• another repeat sample specimen should be collected 90 days following the last RBC
transfusion.
If the first sample specimen is collected prior to RBC transfusion but less than 24 hours of life (early
screen):
• a repeat sample specimen should be collected at 28 hours post transfusion start time.
• another repeat sample should be collected at discharge or 30 days of life, whichever comes
first.

17
Early Specimens
Any specimen collected at less than 24 hours of life is considered an early specimen. A repeat
specimen is required as soon as possible, but no later than two weeks of life. Certain clinical
circumstances require obtaining a specimen at less than 24 hours of life.
Collect an early specimen prior to:
• Red blood cell transfusion
• Surgery
• Initiating extracorporeal membrane oxygenation (ECMO)
• Transfer to another hospital
If a newborn is unlikely to survive the first 24 hours of life, a NBS specimen should be collected at the
parent’s discretion.
Total Parenteral Nutrition (TPN)
TPN affects the acylcarnitine and amino acid profiles for the amino acid, fatty acid oxidation and
organic acid disorders. However, after several years of tandem mass spectrometry experience, the
Michigan NBS Laboratory has detected several cases of each of these disorders in newborns on
TPN feeding. There have been no known false negatives. As a result of this experience and the
complexities involved in devising a screening algorithm that would obtain a TPN free specimen for all
newborns, the Michigan NBS Program does not include TPN status in the NICU screening algorithm.
For NICU newborns on TPN who test positive, repeat specimens are requested using the General
Elevation of Acylcarnitines or Amino Acids letter.
Transferred Newborns
• The birth hospital is responsible for ensuring that NBS specimens are collected on all
newborns, regardless of age, and sent to the NBS Laboratory.
• The birth hospital should notify the receiving facility of the NBS status and include verification
of screening in the transport paperwork.
• The birth hospital should write ‘transferred to’ and the name of the receiving hospital in the
Notes section at the bottom of the card.
• The receiving hospital should verify the screening status of all transferred newborns. If
screening cannot be verified, the receiving hospital should obtain the newborn screen.
• If the newborn screen was done prior to 24 hours of life, the receiving hospital should do the
24-36 hour and 30-day or discharge screens as with other NICU newborns.
• Some results are valid on early specimens (hemoglobin, galactosemia) and obtaining this
specimen will avoid the request for a 90-day specimen if there is a transfusion before a
second specimen is obtained.
• Each state has different NBS policies. If you admit a newborn transferred from another state,
you should try to obtain the screening status from the birth facility. If screening status cannot
be verified, collect a newborn screen. If a Michigan newborn is transferred to another state, a
NBS specimen should be obtained prior to transfer.

18
Newborn at High Risk of Having a NBS Disorder
The NBS Program should be notified by telephone (517-335-4181) if a newborn or a newborn’s
sibling is suspected of having a NBS disorder. This information can also be included in the Notes
section on the card.
Newborn Death or Pending Death
The NBS Program should be notified if a newborn has died or is expected to die. A NBS specimen
should be obtained at the parent’s discretion to determine if the newborn has a NBS disorder. This
information is important for parents in planning future pregnancies. Notify the NBS Follow-up
Program (fax 517-335-9419 or 517-335-9739) when a death is expected or occurs. This will prevent
unnecessary notification of parents regarding subsequent screening or diagnostic testing.

19
PRIMARY CARE PROVIDER INFORMATION
Follow-up of Positive NBS Results
When the NBS Program identifies a strong positive NBS result, the primary care provider is
immediately notified by fax. The following items are included in the fax notification:
• NBS results
• Action required
• Sub-specialist contact information
Simultaneously, the appropriate sub-specialist is notified. The primary care provider will be contacted
by the consulting sub-specialist to develop a plan of action for necessary diagnostic testing and
evaluation that is congruent with clinical status.
The NBS Program may ask the primary care provider or hospital for additional information over time
as part of program evaluation and long-term follow-up. The requests for information are required for
NBS follow-up and are not subject to limitations of the
Health Information Portability and
Accountability Act (HIPAA).
NBS Result Request Policy
The NBS Program does not give NBS results over the telephone.
NBS results are available on the Michigan Care Improvement Registry
website after the NBS record
is successfully linked to the newborn’s birth certificate and immunization record. The NBS results are
not posted if the baby has been released for adoption or placed in foster care.
Alternatively, if the laboratory has completed testing, results can be obtained by faxing a request on
primary care provider letterhead to 517-335-9419 or 517-335-9739. The request should include:
• Baby’s name and date of birth
• Mother’s name at time of delivery
• Primary care provider fax number
• If you are not the provider recorded on the NBS card, the NBS Report Request Form
must be
completed, signed by the medical provider, and faxed to the number listed above. The form is
in
appendix 13.
If results for a particular test needed, please specify the disorder on your request.
Questions on Positive Reports Received
If you receive a positive report for a baby and have questions, contact the NBS follow-up
coordinating center listed on the letter.
The following are common calls received by the NBS Program for information that is available on the
NBS website or in this guide:
• List of disorders included in the Michigan NBS panel
• NBS follow-up coordinating centers
• Written instructions for completing the NBS card and specimen collection instructions
• NBS annual reports that give the number of confirmed cases per year

20
Documentation of NBS Results
Documentation that a newborn has been screened should be available for every newborn and
included in the medical record.
The NBS Program recommends that a log be kept of each blood spot screen collected. The log
should include the following information:
• Baby’s demographic information
• Mother’s name and birth date
• NBS card (“kit”) number
• Barcode number on the NBS envelope in which each screen was placed
A separate courier log should be kept that includes:
• The date the NBS envelope was prepared
• The barcode number of each NBS envelope
• A place for the courier to sign and date the log
The submitter of the NBS first sample, usually the hospital or homebirth attendant, should have a
mechanism in place to track NBS results. Do not assume that no news is always good news. If you
cannot locate NBS results, verify that the screening was done. If results are not received within two
weeks following sample submission, first contact your hospital laboratory and/or medical records
department for results or contact the birth hospital for newborns transferred to your hospital. Check
the Michigan Care Improvement Registry
(MCIR) website for NBS results. If the NBS results cannot
be found, contact the NBS Follow-up Program via phone at 517-335-4181 to obtain a copy of the
results.
The hospital (newborn remains hospitalized) or primary care provider (newborn has been
discharged) are responsible for facilitating the collection of a repeat sample as needed. Repeat
samples are requested whenever a first sample specimen is borderline positive, unsatisfactory for
testing, inconclusive, collected before 24 hours of life or collected after RBC transfusion.
Missing Bloodspot Newborn Screens
Every year, approximately 40-50 hospital births have a missed newborn blood spot screen, which
delays potential identification of life-threatening disorders. Each week the NBS Program staff link
electronic birth certificate (EBC) records to newborn screening specimens received. This process
enables quick identification of infants with a Michigan birth certificate, but no record of a blood spot
screen. Hospitals are alerted of these potentially missed newborn screens.
After the linkage, a manual review occurs for all infants not listed as deceased who show as having
no blood spot on file. This includes a search of the NBS database and review of faxes received from
hospitals documenting why a screen was not collected. If explanatory records are not found, NBS
Program staff send a “No Record of Blood Spot Screen” fax to the designated NBS coordinator at the
birth facility. A request is made for additional information regarding the reason for the missing blood
spot, such as if the infant died before a screen was collected, infant was transferred out of state,
name change due to adoption, etc. If the screen was truly missed, the NBS Program requests that
the birth facility contact the family to return to have a screen collected as soon as possible.

21
Due to the critical importance of identifying affected infants and initiating treatment as quickly as
possible, the NBS Program implemented the following process to ensure timely response. If
acknowledgment that the screen was missed and/or a reason for no blood spot collection is received,
the further actions in the timeline will end. Appendix 15
contains a detailed process timeline.
• Day One: Initial fax sent to birth facility’s NBS Coordinator.
• Day Two: NBS staff calls to ensure the fax has been received.
• Day Three: Second attempt fax is sent to NBS Coordinator.
• Day Five: MDHHS NBS nurse consultant calls NBS Coordinator to review case and gather
information.
• Day Seven: Letters will be sent to the parents letting them know that the NBS Program has
not received a screen for their infant and to the risk management area at the birth facility.
It is ultimately the responsibility of the birth facility to ensure that all infants receive a
newborn screen. Prevention of missed screenings is critical. Using a NBS specimen log is one
method to track that collection takes place. Proactively informing the MDHHS NBS Program of
reasons for not collecting a NBS can be completed utilizing NBS Follow-up Program Hospital
Discharge Sheet found in Appendix 7
. If a parent will not permit collection, please fax the signed
copy of your institution’s refusal form to the MDHHS NBS Follow-up Program at 517-335-9419 or
517-335-9739.

22
FREQUENTLY ASKED QUESTIONS
Who informs parents about NBS?
Although education is ideally done during the prenatal period, the birth hospital is ultimately
responsible for informing parents about the NBS process. To facilitate talking with parents, the NBS
Program recommends using the Michigan Newborn Screening Saves Babies parent brochure and
the Newborn Screening Roadmap Infographic as tools. NBS materials may be ordered on the
newborn screening ordering website. Additional information is available on the newborn screening
website.
What is the chance that a newborn will have a disorder detected by NBS?
Of the 106,126 infants screened in 2019, 284 were diagnosed with a disorder. Overall, one infant out
of 374 screened was diagnosed with one of the disorders included in the Michigan NBS panel.
What if a newborn has a family history of a disorder detected by NBS?
Please inform the NBS Program if a family has a history of a disorder on the Michigan NBS panel.
You may write this information in the Notes section of the NBS card or call the NBS Follow-up
Program at 517-335-4181.
What is the NBS Program’s specimen storage policy?
Residual NBS specimens are stored for up to 100 years once NBS is completed. Stored specimens
may be used for quality control purposes or for new test development. Medical or public health
researchers may use coded specimens through the Michigan BioTrust for Health once their proposal
has been reviewed and approved by the BioTrust Scientific Advisory Board and the MDHHS
Institutional Review Board. NBS specimens collected after May 1, 2010 can only be used if parental
consent is granted for such research. Specimens collected prior to May 1, 2010 are available for
research unless parents contact the MDHHS and opt-out using the form in Appendix 9
.
Who decides what disorders are included on the NBS panel?
The legislatively mandated Quality Assurance Advisory Committee makes recommendations on
disorder inclusion to the MDHHS director, typically following recommendations from the federal
Advisory Committee on Heritable Disorders in Newborns and Children.
Based on nationally accepted criteria, the NBS Quality Assurance Advisory Committee makes
recommendations on disorder inclusion to the MDHHS director. The NBS Quality Assurance
Advisory Committee meets once each year. Members include parents of affected children,
healthcare providers, hospital representatives, and other medical experts.
What if I need to talk to someone at the NBS Program or a medical sub-specialist?
Call 517-335-4181 to reach someone in the NBS Program. You may also call the appropriate
NBS
follow-up coordinating center to speak to a sub-specialist.

23
RESOURCE LIST
Newborn Screening Links
Website URL
Michigan Newborn Screening
Michigan.gov/newbornscreening
Michigan Newborn Screening Online
(Order placement website)
Michigan.gov/nbsorders
Michigan Critical Congenital Heart Disease
Newborn Screening Program
Michigan.gov/cchd
Michigan BioTrust for Health Parental
Consent Process Training
Michigan.gov/biotrust
Genetics Home Reference
medlineplus.gov/genetics
Centers for Disease Control and
Prevention Genomics Resources
cdc.gov/genomics/resources
Newborn Screening Course
https://courses.mihealth.org/PUBLIC/home.html
American Academy of Pediatrics
aap.org
Sickle Cell Disease Association of America
– Michigan Chapter, Inc.
scdaami.org
National Newborn Hearing Links
Website URL
Centers for Disease Control Early Hearing
Detection and Intervention
cdc.gov/ncbddd/hearingloss/ehdi-data.html
Marion Downs Center
mariondowns.org
National Institute on Deafness and Other
Communication Disorders
nidcd.nih.gov
American Speech-Language-Hearing
Association
asha.org
American Academy of Audiology
audiology.org
Hands and Voices
handsandvoices.org

24
CONTACT INFORMATION
NBS Follow-up
NBS
Follow-up
Program
MDHHS
Newborn Screening
Follow-up
333. S. Grand Ave.,
2
nd
floor
PO Box 30195
Lansing, MI 48909
Toll-free:
866-673-9939
Telephone:
517-335-4181
Fax:
517-335-9419 or
517-335-9739
Email:
NewbornScreening@Michigan.gov
Website:
Michigan Newborn Screening
Staff
Title
Phone Number
Contact for questions
regarding:
Mary Kleyn
NBS Follow-up
section manager
517-335-9296
Destroying leftover NBS
specimens
Angela Aldrich
NBS nurse
consultant
517-335-1966
NBS hospital education
NBS collection
Quality metrics performance
Becky Shaulis
NBS Quality
Assurance
specialist
517-335-8532
Preliminary results request due to
clinical concerns
Reporting updated hospital staff
contact information
Lacey
VanLoenen
NBS operations
coordinator
517-335-1207
Courier issues
Valerie Ewald
NBSO technical
administrator
517-335-1400
NBS card orders
NBS educational brochure orders
Kristen
Thompson
NBS program
coordinator
517-284-4992
CCHD
NBS parent education
Shelby Atkinson
Genomics and NBS
Research
Coordinator
517-335-6497
BioTrust for Health

25
CONTACT INFORMATION
NBS Lab
NBS
Laboratory
MDHHS
Newborn Screening Laboratory
3350 N. Martin Luther King Blvd.
PO Box 30689
Lansing, MI 48909
Telephone:
517-241-6366
Fax:
517-335-9773
Staff
Title
Phone Number
Vacant
NBS Laboratory Section
Manager
517-241-6366
Shawn Moloney
NBS Laboratory Metabolic Unit
Manager
517-335-5097
Joseph Hill
NBS Laboratory Endocrine Unit
Manager
517-335-9381
Alayna Bunker
Departmental technician
517-335-4031

26
CONTACT INFORMATION
Early Hearing Detection and Intervention
Hearing
MDHHS
Early Hearing Detection and
Intervention
109 W. Michigan Ave., 3
rd
floor
PO Box 30195
Lansing, MI 48909
Telephone:
517-335-8955
Fax:
517-763-0183
Website:
Michigan Early Hearing Detection and
Intervention
Staff
Title
Phone Number
Nick Drzal
Infant Health Unit manager
517-241-1914
Erin Estrada
Data analyst
517-335-8916
Michelle Garcia
Follow-up consultant
517-335-8878
Krystina
Trowbridge
Infant Health Unit secretary
517-335-8955
Gina Cooper
EHDI coordinator
517-335-4941
Nan Asher
Program consultant
517-335-8273
Dona McGovern
EHDI administrative assistant
517-335-7835

27
CONTACT INFORMATION
NBS Hepatitis B
Hepatitis B
MDHHS
Perinatal Hepatitis B Prevention
Program
333 S. Grand Ave., 3
rd
floor
PO Box 30195
Lansing, MI 48909
Telephone:
517-242-8319
Fax:
517-763-0470
Website:
Perinatal Hepatitis B Prevention Program
Staff
Title
Phone Number
Pat Fineis
Program coordinator
517-242-8319
Marcy Smith
Case manager/Out-state
517-388-4815
Naomi
Scherman-Siver
Case manager/SE MI
517-897-3236

28
CONTACT INFORMATION
NBS Follow-up Coordinating Centers
Hemoglobinopathies
Sickle cell anemia (Hb SS), hemoglobin
SC disease, sickle beta thalassemia
zero (Sβ
0
), sickle beta thalassemia plus
(Sβ+), and hemoglobin H disease.
Sickle Cell Disease Association
of America, Michigan Chapter
18516 James Couzens
Detroit, MI 48235
Telephone: 313-864-4406
Toll-free: 800-842-0973
Fax: 313-864-9980
info@scdaami.org
Metabolic Disorders
Amino acid disorders, fatty acid
oxidation disorders, organic acid
disorders, galactosemia, biotinidase
deficiency
Children's Hospital of Michigan
Metabolic Clinic
3950 Beaubien Blvd.
Detroit, MI 48201-2192
Telephone: 313-832-9330
Fax: 313-745-8030
Lysosomal Storage
Disorders (LSD)
Pompe Disease &
Mucopolysaccharidosis Type (MPSI)
Children's Hospital of Michigan
Genetics Clinic
3950 Beaubien Blvd.
Detroit, MI 48201-2192
Telephone: 313-832-9330
Fax: 313-745-8030
Michigan Medicine at the
University of Michigan
Division of Pediatric Genetics,
Metabolism, and Genomic
Medicine
D5240 Medical Professional Bldg.
1500 E. Medical Center Drive
Ann Arbor, MI 48109-5718
Telephone: 734-764-0579
Fax: 734-763-6561
Endocrine Disorders
Cystic Fibrosis (CF)
Spinal Muscular
Atrophy (SMA)
X-linked
Adrenoleukodystrophy
(X-ALD)
Congenital adrenal hyperplasia (CAH)
Congenital hypothyroidism (CH),
CF
X-ALD
SMA
Michigan Medicine at the
University of Michigan
Department of Pediatrics
1500 E. Medical Center Dr.
D1225 MPB, Box 5718
Ann Arbor, MI 48109-0718
Telephone: 734-647-8938
Fax: 734-936-7918
Primary
Immunodeficiency
Disorders
Severe combined immunodeficiency
disorder (SCID) and other primary
immunodeficiency disorders with T-
cell lymphopenia
3950 Beaubien St.
Detroit, MI 48201
Telephone: 313-806-6571
Pager: 313-745-0203; pager
number 5706
Fax: 313-966-9701
29
CONTACT INFORMATION
Courier Services
Lower Peninsula
Monday-Friday and Sunday pickup, including holidays.
Hospitals will be notified each time a Sunday pickup schedule will be followed for holidays that fall on
a weekday.
STAT Courier Services, Inc.
Toll-fee: 888-592-7828
Tresa Agee, Customer Care / Account Manager
Email: tagee@stat-courier.com
Michigan account number: 995
Upper Peninsula
Monday-Saturday pickup
United Parcel Services
Toll-free: 800-877-1797
Online tracking: UPS tracking
website
Use account number 05V0R4 when ordering UPS Medical Envelopes
Email NewbornScreening@Michigan.gov for UPS shipping labels
Contact NewbornScreening@Michigan.gov if you need detailed NBS courier information such as
your courier pickup time, days and location.

30
APPENDIX 1 – LEGISLATIVE MANDATES
Public Health Code Act 368 of 1978
The NBS Program applies to all newborns in the State of Michigan by law. You can find the law in its
entirety online on the Public Health Code Act 368 of 1978 website.
Some highlights are:
• Health professional in charge of the care of a newborn infant or, if none, the health
professional in charge at the birth of an infant must collect the newborn screen
• List of disorders screened
• Informed consent of the parent is not required
• Positive results shall be reported to the infant’s parents, guardian or person in loco parentis
• NBS fee and adjustment
• Hardship waiver of the fee is authorized
• Retention and disposal schedule are established
Act No. 31, Public Acts of 2006 to amend 1978 PA 368
This amendment can be found in its entirety on the Act No. 31, Public Acts of 2006 to amend 1978
PA 368 website.
Some highlights are:
• Creation of the newborn screening quality assurance advisory committee
o Committee members
o Review disorders screened and recommend new disorders for addition to the
screening panel
o Financial review of the NBS Program with recommendation to adjust the amount
charged
• Addition of screening for hearing loss

31
APPENDIX 2 – BLOOD SPECIMEN COLLECTION AND HANDLING PROCEDURE
These instructions are found on the back of each NBS kit:
Please follow the Clinical and Laboratory Standards Institute (CLSI) guidelines for NBS specimen
collection. Refer to the Clinical and Laboratory Standards Institute website for additional information.

32
APPENDIX 3 – NBS CARD IMAGES
NBS First Sample (“blue”) Card
Michigan BioTrust for Health Consent Form (Attached to the back of the NBS first sample card)

33
APPENDIX 4 – HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT
(HIPAA) PRIVACY RULE
The HIPAA Privacy Rule recognizes the need for public health programs to access protected health
information (PHI) to conduct public health activities to prevent or control disease, injury or disability.
The Privacy Rule expressly permits release of PHI relating to newborn screening, without individual
authorization, from a covered entity to state public health departments or agencies contacted, by
public health departments, to provide newborn screening follow-up.
The Privacy Rule can be found in its entirety on the HIPAA Privacy Rule and Public Health
website.

34
APPENDIX 5 – NBS ONLINE ORDERING SYSTEM (NBSO)
NBS brochures and mailing envelopes are available at no charge. NBS cards need to be purchased
when the order is placed.
NBSO Payment Options: The NBSO system allows you to enter your hospital’s purchase order
number and assign it to your order. Payment options include invoice, eCheck or credit card. These
payments will be processed through the PayPlace, a secure site used by the State of Michigan for
financial transactions. Learn more about PayPlace privacy on the PayPlace privacy website. Invoice
orders and eCheck orders will require a unique verification code. Please call 517-335-1400 to get
your permanent verification code, which you will need to use for all future orders. There is an
annually adjusted processing fee of approximately 2.8% added to each credit card purchase.
eChecks are an efficient and secure form of payment. They are used just like a check, but the bank
routing number and account number will be entered electronically on the web-based order form
instead of on a paper check. eCheck is NOT the same as an electronic fund transfer (EFT). An
eCheck is processed like a check and the account is not debited until the check clears. Learn more
on the eCheck website.
If you are using a credit card, make sure the approved credit limit on the card is sufficient to cover the
full cost of cards being purchased, and that the name, billing address and zip code associated with
the credit card account match the information you enter in PayPlace.
Follow these steps to get started:
1. Visit Michigan.gov/nbsorders
2. Click NBSOnline Web Store – Order NOW!
3. Click Register if you don’t have an account.
4. If you are ordering for multiple medical facilities, click on each of the hospitals for which you
do purchasing – a check mark will appear.
5. Upon completion of the registration process, a link will be sent to the email address used to
register.
Still have questions? Please email NBSOrders@Michigan.gov
or call 517-335-1400.

35
APPENDIX 6 – NEWBORN SCREENING CARD REPLACEMENT FORM
Date:
Facility name:
Attention/Department:
Address:
City, state, zip:
Contact name: Telephone:
Number of cards returned for replacement:
ID number(s) of the card(s) returned:
• This form should be filled out completely and mailed with the white face sheet(s) only of the
card(s) intended for replacement to the address below. It is not necessary to include the
remaining portions of the kit.
• If there is blood on the white face sheet, place it in a biohazard bag.
• DO NOT send card replacement requests to the NBS Laboratory. Failure to send your
request to the address below may result in no replacement card being issued. Please note:
Courier envelopes are for blood spot specimens. DO NOT use courier envelopes for card
replacement requests.
SEND FACE SHEET(S) OF CARD(S) TO BE REPLACED AND THIS FORM TO:
Michigan Department of Health and Human Services
Attention: Newborn Screening
333 S. Grand Ave., 2
nd
floor
PO Box 30195
Lansing, MI 48909
Rev. 01-01-2017

36
APPENDIX 7 – NBS FOLLOW-UP PROGRAM HOSPITAL DISCHARGE SHEET
Phone 517-335-4181 or 877-673-9939
Fax updated information to: 517-335-9419 or 517-335-9739
Place infant’s hospital label here:
OR
Print infant’s information here:
Name:
DOB:
Circle gender:
boy
girl
ambiguous
NBS card (kit) number:
Hospital name:
NBS hospital code:
Check nursery type: NICU: ___ Regular: ___ Special Care: ___
Name of hospital infant transferred to:
Infant’s primary care provider (PCP):
(Will care for infant after discharge)
PCP phone:
PCP fax:
Provide guardian contact information if infant is not released to the care of the mother:
Guardian name:
Guardian address:
Guardian phone:
Name of staff person completing form (please print):
Date:
Phone:
Fax:
Notes:
(e.g., parent would not permit NBS, infant has meconium ileus, sibling has cystic fibrosis, etc.)

37
APPENDIX 8 – DISORDER LIST
The Newborn Screening Laboratory screens all Michigan infants for more than fifty disorders.
Amino Acid Disorders
1. Argininemia (ARG)
2. Argininosuccinic acidemia (ASA)
3. Citrullinemia Type I (CIT-I)
4. Citrullinemia Type II (CIT-II)
5. Homocystinuria (HCY)
6. Hypermethioninemia (MET)
7. Maple syrup urine disease (MSUD)
8. Phenylketonuria (PKU)
9. Benign hyperphenylalaninemia defect
(H-PHE)
10. Biopterin cofactor biosysnthesis defect
(BIOPT-BS)
11. Biopterin cofactor regeneration defect
(BIOPT-REG)
12. Tyrosinemia Type I (TYR-1)
13. Tyrosinemia Type II (TYR-II)
14. Tyrosinemia Type III (TYR-III)
Fatty Acid Oxidation Disorders
15. Carnitine acylcarnitine translocase deficiency
(CACT)
16. Carnitine palmitoyltransferase I deficiency
(CPT-1A)
17. Carnitine palmitoyltransferase II deficiency
(CPT-II)
18. Carnitine uptake defect (CUD)
19. Dienoyl-CoA reductase deficiency (DERED)
20. Glutaric acidemia type II (GA-2)
21. Long-chain L-3-hydroxy acyl-CoA dehydrogenase
deficiency (LCHAD)
22. Medium/short-chain L-3-hydroxy acyl-CoA
dehydrogenase deficiency (M/SCHAD)
23. Medium-chain acyl-CoA dehydrogenase deficiency
(MCAD)
24. Medium-chain ketoacyl-CoA thiolase deficiency
(MCKAT)
25. Trifunctional protein deficiency (TFP)
26. Very long-chain acyl-CoA dehydrogenase
deficiency (VLCAD)
Organic Acid Disorders
27. 2-Methyl-3-hydroxy butyric aciduria (2M3HBA)
28. 2-Methylbutyryrl-CoA dehydrogenase deficiency
(2MBG)
29. 3-hydroxy 3-methylglutaric glutaric aciduria (HMG)
30. 3-Methylcrotonyl-CoA carboxylase deficiency
(3-MCC)
31. 3-Methylglutaconic aciduria (3MGA)
32. Beta-ketothiolase deficiency (BKT)
33. Glutaric acidemia type I (GA1)
34. Isovaleric acidemia (IVA)
35. Malonic Acidemia (MAL)
36. Methylmalonic acidemia cobalamin disorders
(Cbl A,B)
37. Methylmalonic aciduria with homocystinuria
(Cbl C,D)
38. Methylmalonic acidemia methylmalonyl-CoA mutase
(MUT)
39. Multiple carboxylase deficiency (MCD)
40. Propionic acidemia (PROP)
Hemoglobinopathies
41. S/Beta thalassemia
42. S/C disease
43. Sickle cell anemia
44. Variant hemoglobinopathies
45. Hemoglobin H disease
Endocrine Disorders
46. Congenital adrenal hyperplasia (CAH)
47. Congenital hypothyroidism (CH)
Lysosomal Storage Disorders
48. Glycogen storage disease type II (Pompe)
49. Mucopolysaccharidosis type I (MPS I)
Other Disorders
50. Biotinidase deficiency (BIOT)
51. Galactosemia (GALT)
52. Cystic fibrosis (CF)
53. Severe combined immunodeficiency (SCID)
54. T-cell related lymphocyte deficiencies
55. X-linked adrenoleukodystrophy (X-ALD)
56. Hearing
57. Critical congenital heart disease (CCHD)
58. Spinal muscular atrophy
Disorder Coming Soon
The following conditions have been approved for
addition to Michigan’s panel, but implementation is in
progress and screening has not yet begun.
• Guanidinoacetate methyltransferase (GAMT)
deficiency
Updated April 2020
39
APPENDIX 10 – NICU AND SPECIAL CARE NURSERY ALGORITHM
NICU SCREENING ALGORITHM
For All NICU Newborns NOT Transfused
FIRST SPECIMEN
24-30 hours of life
SECOND SPECIMEN
30 days of life or if discharged between 8-29 days of life
Whichever comes first
For All NICU Newborns Transfused with Red Cells
PRIOR to First Specimen
Transfused
FIRST SPECIMEN
28 hours post transfusion start
SECOND SPECIMEN
30 days of life or if discharged between 8-29 days of life
Whichever comes first
THIRD SPECIMEN
90 days post last transfusion
For All Newborns Transfused with Red Cells
AFTER First Specimen
FIRST SPECIMEN
>= 24 hours of life
Transfused
SECOND SPECIMEN
30 days of life or if discharged
between 8-29 days of life
Whichever comes first
OR
FIRST SPECIMEN
< 24 hours of life
Transfused
SECOND SPECIMEN
28 hours post transfusion start
THIRD SPECIMEN
30 days of life or if discharged
between 8-29 days of life
Whichever comes first

40
APPENDIX 11 – FAX REPORTING
The Michigan Department of Health and Human Services (MDHHS) encourages the receipt of
Newborn Screening (NBS) laboratory reports via an automatic fax transmission. Fax reporting
provides significant improvement in screening result turnaround time to your practice. A secure fax
must be available 24 hours per day, 7 days per week because fax reporting can occur anytime
during the day or night, including weekends. Expect the same number of pages per patient as are
currently mailed. Faxes that fail to get through after several automatic redial attempts will be resent
promptly.
If your hospital chooses this fax reporting option, the delivery of NBS laboratory reports through the
United States Postal System will be eliminated.
Please notify MDHHS NBS Laboratory if your fax machine is down for repairs.
• If an alternate, secure fax number is available, reporting can be promptly changed to the
alternate fax.
• If you no not have an alternate secure fax, reports will be mailed until you notify the MDHHS
NBS Laboratory that your machine is again operational.
You must meet two requirements to receive newborn screening results by fax:
1. A letter on your hospital letterhead must be sent to the MDHHS Bureau of Laboratories,
Newborn Screening, consenting to receive automatic faxed results. The letter must be
signed by a person who is authorized to make this request.
2. The statement of understanding (next page) must be completed, signed and returned with
the consenting letter.
Send both to:
MDHHS Newborn Screening Laboratory
3350 N. Martin Luther King Blvd.
PO Box 30689
Lansing, MI 48909
OR Fax: 517-335-8550
Direct any questions to Alayna Bunker, 517-335-4031, email BunkerA@michigan.gov
;
or Jenny Kramer, 517-335-8095, email KramerJ8@michigan.gov
41
STATEMENT OF UNDERSTANDING
PRACTICES SELECTING AUTOMATIC FAX TRANSMISSION OPTION
1. I understand that all newborn screening reports of patient testing by the MDHHS Bureau of
Laboratories will be sent to this practice by fax transmission.
2. I understand that upon conversion to a fax transmission agency, no hard copy reports will be
sent using the United States Postal Service.
3. The fax number provided to MDHHS is a secure facsimile machine. To be a secure
facsimile machine, the following criteria must be met:
Only persons authorized to review confidential clinical laboratory test results use or
otherwise have access to incoming fax transmissions.
The facsimile machine is in a secure location during non-business hours in the event
that fax transmittal occurs outside of normal business hours.
Date:
Hospital name:
Address:
Authorized person (please print):
Authorized signature:
Secure fax number:
Contact person for fax problems (please print):
Contact person’s phone number:
Please keep a copy for your records

42
APPENDIX 12 – MILITARY TIME
Military time is a concise method of expressing time used by the military, law enforcement, hospitals,
and other entities. Military time uses a 24-hour time scale that makes the use of a.m. or p.m.
unnecessary. Midnight corresponds to 0000, 1 p.m. corresponds to 1300, and so on.
Check to see if your smart phone can default to 24-hour time. Follow these easy steps to change
your iPhone default time:
1. From the iPhone’s home screen, tap Settings.
2. Tap General.
3. Scroll to the bottom of the screen and tap Date and Time.
4. Move the 24-hour time slider to the “on” position.
5. Your iPhone will now display the 24-hour clock at the top of the screen and in the Clock
application.

44
APPENDIX 14 – WEIGHT CONVERSION CHART

45
APPENDIX 15 – MISSING BLOODSPOT SPECIMEN PROCESS


